Combinatorial cysteine mutagenesis reveals a critical intramonomer role for cysteines in prestin voltage sensing
- PMID: 20655836
- PMCID: PMC2895379
- DOI: 10.1016/j.bpj.2010.03.066
Combinatorial cysteine mutagenesis reveals a critical intramonomer role for cysteines in prestin voltage sensing
Abstract
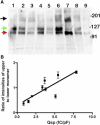
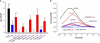
Prestin is a member of the SLC26 family of anion transporters and is responsible for electromotility in outer hair cells, the basis of cochlear amplification in mammals. It is an anion transporting transmembrane protein, possessing nine cysteine residues, which generates voltage-dependent charge movement. We determine the role these cysteine residues play in the voltage sensing capabilities of prestin. Mutations of any single cysteine residue had little or no effect on charge movement. However, using combinatorial substitution mutants, we identified a cysteine residue pair (C415 and either C192 or C196) whose mutation reduced or eliminated charge movement. Furthermore, we show biochemically that surface expression of mutants with markedly reduced functionality can be near normal; however, we identify two monomers of the protein on the surface of the cell, the larger of which correlates with surface charge movement. Because we showed previously by Förster resonance energy transfer that monomer interactions are required for charge movement, we tested whether disulfide interactions were required for dimerization. Using Western blots to detect oligomerization of the protein in which variable numbers of cysteines up to and including all nine cysteine residues were mutated, we show that disulfide bond formation is not essential for dimer formation. Taken together, we believe these data indicate that intramembranous cysteines are constrained, possibly via disulfide bond formation, to ensure structural features of prestin required for normal voltage sensing and mechanical activity.
Copyright 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Zheng J., Madison L.D., Dallos P. Prestin, the motor protein of outer hair cells. Audiol. Neurootol. 2002;7:9–12. - PubMed
-
- Liberman M.C., Gao J., Zuo J. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature. 2002;419:300–304. - PubMed
-
- Brownell W.E., Bader C.R., de Ribaupierre Y. Evoked mechanical responses of isolated cochlear outer hair cells. Science. 1985;227:194–196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

