Drug sensitivity, drug-resistant mutations, and structures of three conductance domains of viral porins
- PMID: 20655872
- PMCID: PMC3709975
- DOI: 10.1016/j.bbamem.2010.07.015
Drug sensitivity, drug-resistant mutations, and structures of three conductance domains of viral porins
Abstract
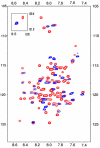
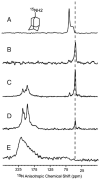
Recent controversies associated with the structure of the M2 protein from influenza A virus and the binding site of drug molecules amantadine and rimantadine motivated the comparison here of the drug binding to three viral porins including the M2 proteins from influenza A and B as well as the viral protein 'u' from HIV-1. While the M2 protein from influenza B does not normally bind amantadine, chimeras with the M2 protein from influenza A show blockage by amantadine. Similarly, Vpu does not normally bind rimantadine, but the single site mutation A18H converts a non-specific channel to a selective proton channel that is sensitive to rimantadine. The comparison of structures and amino acid sequences shows that the membrane protein sample environment can have a significant influence on the structural result. While a bilayer surface bound amphipathic helix has been characterized for AM2, such a helix may be possible for BM2 although it has evaded structural characterization in detergent micelles. A similar amphipathic helix seems less likely for Vpu. Even though the A18H Vpu mutant forms rimantadine sensitive proton channels, the binding of drug and its influence on the protein structure appears to be very different from that for the M2 proteins. Indeed, drug binding and drug resistance in these viral porins appears to result from a complex set of factors.
2010 Elsevier B.V. All rights reserved.
Figures






References
-
- Hout DR, Gomez LM, Pacyniak E, Miller JM, Hill MS, Stephens EB. A single amino acid substitution within the transmembrane domain of the human immunodeficiency virus type 1 Vpu protein renders simian-human immunodeficiency virus (SHIV(KU-1bMC33)) susceptible to rimantadine. Virology. 2006;348:449–461. - PubMed
-
- Hout DR, Gomez ML, Pacyniak E, Gomez LM, Fegley B, Mulcahy ER, Hill MS, Culley N, Pinson DM, Nothnick W, Powers MF, Wong SW, Stephens EB. Substitution of the transmembrane domain of Vpu in simian–human immunodeficiency virus (SHIVKU1bMC33) with that of M2 of influenza A results in a virus that is sensitive to inhibitors of the M2 ion channel and is pathogenic for pig-tailed macaques. Virology. 2006;344:541–559. - PubMed
-
- Pinto LH, Lamb RA. Influenza virus proton channels. Photochem. Photobiol. Sci. 2006;5:629–632. - PubMed
-
- Mould JA, Paterson RG, Takeda M, Ohigashi Y, Venkataraman P, Lamb RA, Pinto LH. Influenza B virus BM2 protein has ion channel activity that conducts protons across membranes. Dev. Cell. 2003;5:175–184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

