Modulation of host cell responses and evasion strategies for porcine reproductive and respiratory syndrome virus
- PMID: 20655963
- PMCID: PMC7114477
- DOI: 10.1016/j.virusres.2010.07.019
Modulation of host cell responses and evasion strategies for porcine reproductive and respiratory syndrome virus
Abstract
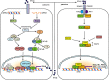
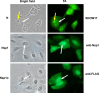
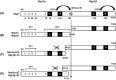
The immune surveillance system protects host cells from viral infection, and viruses have evolved to escape this system for efficient proliferation in the host. Host cells produce cytokines and chemokines in response to viral infection, and among such effector molecules, type I interferons are the principal antiviral cytokines and therefore effective targets for viruses to disarm host surveillance. Porcine reproductive and respiratory syndrome virus (PRRSV) expresses proteins that circumvent the IFN response and other cellular processes, and to compensate the small coding capacity of PRRSV, these proteins are multifunctional. To date, at least four viral proteins have been identified and studied as viral antagonists of host defenses: N as a structural protein and three non-structural proteins, Nsp1 (Nsp1α and Nsp1β), Nsp2, and Nsp11. Among these, N and Nsp1 are nuclear-cytoplasmic proteins distributed in both the nucleus and cytoplasm of cells. Nsp1 and Nsp2 are viral proteases while Nsp11 is an endoribonuclease. This review describes the current understanding of the role of these proteins in modulating the host innate immune responses. Blocking against virus-mediated inhibition of the innate response may lead to the future development of effective vaccines. The understanding of viral mechanisms modulating the normal cellular processes will be a key to the design of an effective control strategy for PRRS.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Interplay between interferon-mediated innate immunity and porcine reproductive and respiratory syndrome virus.Viruses. 2012 Apr;4(4):424-46. doi: 10.3390/v4040424. Epub 2012 Apr 2. Viruses. 2012. PMID: 22590680 Free PMC article. Review.
-
Degradation of CREB-binding protein and modulation of type I interferon induction by the zinc finger motif of the porcine reproductive and respiratory syndrome virus nsp1α subunit.Virus Res. 2013 Mar;172(1-2):54-65. doi: 10.1016/j.virusres.2012.12.012. Epub 2012 Dec 31. Virus Res. 2013. PMID: 23287061
-
Porcine Reproductive and Respiratory Syndrome Virus nsp11 Antagonizes Type I Interferon Signaling by Targeting IRF9.J Virol. 2019 Jul 17;93(15):e00623-19. doi: 10.1128/JVI.00623-19. Print 2019 Aug 1. J Virol. 2019. PMID: 31092569 Free PMC article.
-
Porcine Reproductive and Respiratory Syndrome Virus Nonstructural Protein 1 Beta Interacts with Nucleoporin 62 To Promote Viral Replication and Immune Evasion.J Virol. 2019 Jun 28;93(14):e00469-19. doi: 10.1128/JVI.00469-19. Print 2019 Jul 15. J Virol. 2019. PMID: 31043527 Free PMC article.
-
Modulation of innate immune signaling by nonstructural protein 1 (nsp1) in the family Arteriviridae.Virus Res. 2014 Dec 19;194:100-9. doi: 10.1016/j.virusres.2014.09.007. Epub 2014 Sep 28. Virus Res. 2014. PMID: 25262851 Free PMC article. Review.
Cited by
-
A New Long Noncoding RNA, MAHAT, Inhibits Replication of Porcine Reproductive and Respiratory Syndrome Virus by Recruiting DDX6 To Bind to ZNF34 and Promote an Innate Immune Response.J Virol. 2022 Sep 28;96(18):e0115422. doi: 10.1128/jvi.01154-22. Epub 2022 Sep 8. J Virol. 2022. PMID: 36073922 Free PMC article.
-
Research Progress on NSP11 of Porcine Reproductive and Respiratory Syndrome Virus.Vet Sci. 2023 Jul 10;10(7):451. doi: 10.3390/vetsci10070451. Vet Sci. 2023. PMID: 37505856 Free PMC article. Review.
-
Engineering the PRRS virus genome: updates and perspectives.Vet Microbiol. 2014 Dec 5;174(3-4):279-295. doi: 10.1016/j.vetmic.2014.10.007. Epub 2014 Oct 23. Vet Microbiol. 2014. PMID: 25458419 Free PMC article. Review.
-
Evasion of Antiviral Innate Immunity by Porcine Reproductive and Respiratory Syndrome Virus.Front Microbiol. 2021 Jun 23;12:693799. doi: 10.3389/fmicb.2021.693799. eCollection 2021. Front Microbiol. 2021. PMID: 34512570 Free PMC article. Review.
-
Research progress on the N protein of porcine reproductive and respiratory syndrome virus.Front Microbiol. 2024 Apr 29;15:1391697. doi: 10.3389/fmicb.2024.1391697. eCollection 2024. Front Microbiol. 2024. PMID: 38741730 Free PMC article. Review.
References
-
- Aaronson D.S., Horvath C.M. A road map for those who don’t know JAK-STAT. Science. 2002;296:1653–1655. - PubMed
-
- Akira S., Uematsu S., Tacheuchi O. Pathogenic recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
-
- Albina E., Carrat C., Charley B. Interferon-alpha response to swine arterivirus (PoAV), the porcine reproductive and respiratory syndrome virus. J. Interferon Cytokine Res. 1998;18:485–490. - PubMed
-
- Albina E., Madec F., Cariolet R., Torrison J. Immune response and persistence of the porcine reproductive and respiratory syndrome virus in infected pigs and farm units. Vet. Rec. 1994;134:567–573. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

