Quantitative PCR assay for Mycobacterium pseudoshottsii and Mycobacterium shottsii and application to environmental samples and fishes from the Chesapeake Bay
- PMID: 20656856
- PMCID: PMC2937484
- DOI: 10.1128/AEM.01091-10
Quantitative PCR assay for Mycobacterium pseudoshottsii and Mycobacterium shottsii and application to environmental samples and fishes from the Chesapeake Bay
Abstract
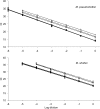
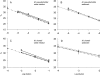
Striped bass (Morone saxatilis) in the Chesapeake Bay are currently experiencing a very high prevalence of mycobacteriosis associated with newly described Mycobacterium species, Mycobacterium pseudoshottsii and M. shottsii. The ecology of these mycobacteria outside the striped bass host is currently unknown. In this work, we developed quantitative real-time PCR assays for M. pseudoshottsii and M. shottsii and applied these assays to DNA extracts from Chesapeake Bay water and sediment samples, as well as to tissues from two dominant prey of striped bass, Atlantic menhaden (Brevoortia tyrannus) and bay anchovy (Anchoa mitchilli). Mycobacterium pseudoshottsii was found to be ubiquitous in water samples from the main stem of the Chesapeake Bay and was also present in water and sediments from the Rappahannock River, Virginia. M. pseudoshottsii was also detected in menhaden and anchovy tissues. In contrast, M. shottsii was not detected in water, sediment, or prey fish tissues. In conjunction with its nonpigmented phenotype, which is frequently found in obligately pathogenic mycobacteria of humans, this pattern of occurrence suggests that M. shottsii may be an obligate pathogen of striped bass.
Figures


References
-
- Blazer, V., W. K. Vogelbein, C. Densmore, D. Zwerner, and E. B. May. 1999. Ulcerative skin lesions in menhaden from Chesapeake Bay. J. Aquat. Anim. Health 11:340-349.
-
- Bruno, D. W., J. Griffiths, C. G. Mitchell, B. P. Wood, Z. J. Fletcher, F. A. Drobniewski, and T. S. Hastings. 1998. Pathology attributed to Mycobacterium chelonae infection among farmed and laboratory-infected Atlantic salmon Salmo salar. Dis. Aquat. Org. 33:101-109. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

