2-haloacrylate hydratase, a new class of flavoenzyme that catalyzes the addition of water to the substrate for dehalogenation
- PMID: 20656877
- PMCID: PMC2937477
- DOI: 10.1128/AEM.00334-10
2-haloacrylate hydratase, a new class of flavoenzyme that catalyzes the addition of water to the substrate for dehalogenation
Abstract
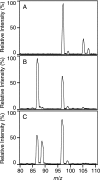
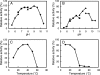
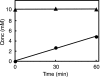
Enzymes catalyzing the conversion of organohalogen compounds are useful in the chemical industry and environmental technology. Here we report the occurrence of a new reduced flavin adenine dinucleotide (FAD) (FADH(2))-dependent enzyme that catalyzes the removal of a halogen atom from an unsaturated aliphatic organohalogen compound by the addition of a water molecule to the substrate. A soil bacterium, Pseudomonas sp. strain YL, inducibly produced a protein named Caa67(YL) when the cells were grown on 2-chloroacrylate (2-CAA). The caa67(YL) gene encoded a protein of 547 amino acid residues (M(r) of 59,301), which shared weak but significant sequence similarity with various flavoenzymes and contained a nucleotide-binding motif. We found that 2-CAA is converted into pyruvate when the reaction was carried out with purified Caa67(YL) in the presence of FAD and a reducing agent [NAD(P)H or sodium dithionite] under anaerobic conditions. The reducing agent was not stoichiometrically consumed during this reaction, suggesting that FADH(2) is conserved by regeneration in the catalytic cycle. When the reaction was carried out in the presence of H(2)(18)O, [(18)O]pyruvate was produced. These results indicate that Caa67(YL) catalyzes the hydration of 2-CAA to form 2-chloro-2-hydroxypropionate, which is chemically unstable and probably spontaneously dechlorinated to form pyruvate. 2-Bromoacrylate, but not other 2-CAA analogs such as acrylate and methacrylate, served as the substrate of Caa67(YL). Thus, we named this new enzyme 2-haloacrylate hydratase. The enzyme is very unusual in that it requires the reduced form of FAD for hydration, which involves no net change in the redox state of the coenzyme or substrate.
Figures


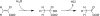
Similar articles
-
2-Haloacrylate reductase, a novel enzyme of the medium chain dehydrogenase/reductase superfamily that catalyzes the reduction of a carbon-carbon double bond of unsaturated organohalogen compounds.J Biol Chem. 2005 May 27;280(21):20286-91. doi: 10.1074/jbc.M414605200. Epub 2005 Mar 21. J Biol Chem. 2005. PMID: 15781461
-
Flavin-N5 Covalent Intermediate in a Nonredox Dehalogenation Reaction Catalyzed by an Atypical Flavoenzyme.Chembiochem. 2018 Jan 4;19(1):53-57. doi: 10.1002/cbic.201700594. Epub 2017 Dec 12. Chembiochem. 2018. PMID: 29116682
-
A mechanistic analysis of enzymatic degradation of organohalogen compounds.Biosci Biotechnol Biochem. 2011;75(2):189-98. doi: 10.1271/bbb.100746. Epub 2011 Feb 7. Biosci Biotechnol Biochem. 2011. PMID: 21307570 Review.
-
A complete bioconversion cascade for dehalogenation and denitration by bacterial flavin-dependent enzymes.J Biol Chem. 2018 Nov 30;293(48):18525-18539. doi: 10.1074/jbc.RA118.005538. Epub 2018 Oct 3. J Biol Chem. 2018. PMID: 30282807 Free PMC article.
-
Role of reduced flavin in dehalogenation reactions.Arch Biochem Biophys. 2021 Jan 15;697:108696. doi: 10.1016/j.abb.2020.108696. Epub 2020 Nov 24. Arch Biochem Biophys. 2021. PMID: 33245912 Review.
Cited by
-
Enzymatic Halogenation and Dehalogenation Reactions: Pervasive and Mechanistically Diverse.Chem Rev. 2017 Apr 26;117(8):5619-5674. doi: 10.1021/acs.chemrev.6b00571. Epub 2017 Jan 20. Chem Rev. 2017. PMID: 28106994 Free PMC article. Review.
-
Comparison of Biochemical Properties of the Original and Newly Identified Oleate Hydratases from Stenotrophomonas maltophilia.Appl Environ Microbiol. 2017 Apr 17;83(9):e03351-16. doi: 10.1128/AEM.03351-16. Print 2017 May 1. Appl Environ Microbiol. 2017. PMID: 28235876 Free PMC article.
-
Structure prediction, molecular dynamics simulation and docking studies of D-specific dehalogenase from Rhizobium sp. RC1.Int J Mol Sci. 2012 Nov 26;13(12):15724-54. doi: 10.3390/ijms131215724. Int J Mol Sci. 2012. PMID: 23443090 Free PMC article.
-
Novel dehalogenase mechanism for 2,3-dichloro-1-propanol utilization in Pseudomonas putida strain MC4.Appl Environ Microbiol. 2012 Sep;78(17):6128-36. doi: 10.1128/AEM.00760-12. Epub 2012 Jun 29. Appl Environ Microbiol. 2012. PMID: 22752160 Free PMC article.
-
Dehalogenases: From Improved Performance to Potential Microbial Dehalogenation Applications.Molecules. 2018 May 7;23(5):1100. doi: 10.3390/molecules23051100. Molecules. 2018. PMID: 29735886 Free PMC article. Review.
References
-
- Banerjee, R., and S. W. Ragsdale. 2003. The many faces of vitamin B12: catalysis by cobalamin-dependent enzymes. Annu. Rev. Biochem. 72:209-247. - PubMed
-
- Bornemann, S. 2002. Flavoenzymes that catalyse reactions with no net redox change. Nat. Prod. Rep. 19:761-772. - PubMed
-
- Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. - PubMed
-
- de Jong, R. M., P. Bazzacco, G. J. Poelarends, W. H. Johnson, Jr., Y. J. Kim, E. A. Burks, H. Serrano, A. M. Thunnissen, C. P. Whitman, and B. W. Dijkstra. 2007. Crystal structures of native and inactivated cis-3-chloroacrylic acid dehalogenase. Structural basis for substrate specificity and inactivation by (R)-oxirane-2-carboxylate. J. Biol. Chem. 282:2440-2449. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

