Preparation of (13)C-labeled ceramide by acetic acid bacteria and its incorporation in mice
- PMID: 20656918
- PMCID: PMC2952581
- DOI: 10.1194/jlr.D009191
Preparation of (13)C-labeled ceramide by acetic acid bacteria and its incorporation in mice
Abstract
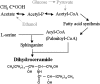
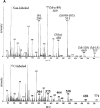
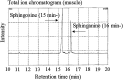
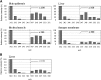
We prepared 2-hydroxypalmitoyl-sphinganine (dihydroceramide) labeled with a stable isotope by culturing acetic acid bacteria with (13)C-labeled acetic acid. The GC/MS spectrum of the trimethylsilyl derivative of (13)C-labeled dihydroceramide gave molecular ions with an increased mass of 12-17 Da over that of nonlabeled dihydroceramide. The fragment ions derived from both sphinganine base and 2-hydroxypalmitate were confirmed to be labeled with the stable isotope in the spectrum. Therefore, (13)C-labeled dihydroceramide can be an extremely useful tool for analyzing sphingolipid metabolism. The purified [(13)C]dihydroceramide was administered orally to mice for 12 days, and the total sphingoid base fractions in various tissues were analyzed by GC/MS. The spectrum patterns specific to (13)C-labeled sphingoids were detected in the tissues tested. Sphinganine pools in skin epidermis, liver, skeletal muscle, and synapse membrane in brain were replaced by [(13)C]sphinganine at about 4.5, 4.0, 1.0, and 0.3%, respectively. Moreover, about 1.0% of the sphingosine pool in the liver was replaced by [(13)C]sphingosine, implying that exogenous dihydroceramide can be converted to sphingosine. These results clearly indicate that ingested dihydroceramide can be incorporated into various tissues, including brain, and metabolized to other sphingolipids.
Figures


Similar articles
-
Elevation of ceramide in Acetobacter malorum S24 by low pH stress and high temperature stress.J Biosci Bioeng. 2010 Jan;109(1):32-6. doi: 10.1016/j.jbiosc.2009.07.007. Epub 2009 Aug 12. J Biosci Bioeng. 2010. PMID: 20129078
-
Biosynthesis of sphingolipids: dihydroceramide and not sphinganine is desaturated by cultured cells.Biochem Biophys Res Commun. 1992 Nov 30;189(1):14-20. doi: 10.1016/0006-291x(92)91518-u. Biochem Biophys Res Commun. 1992. PMID: 1449467
-
Characterization of ceramide synthesis. A dihydroceramide desaturase introduces the 4,5-trans-double bond of sphingosine at the level of dihydroceramide.J Biol Chem. 1997 Sep 5;272(36):22432-7. doi: 10.1074/jbc.272.36.22432. J Biol Chem. 1997. PMID: 9312549
-
Sphingolipid perturbations as mechanisms for fumonisin carcinogenesis.Environ Health Perspect. 2001 May;109 Suppl 2(Suppl 2):301-8. doi: 10.1289/ehp.01109s2301. Environ Health Perspect. 2001. PMID: 11359699 Free PMC article. Review.
-
Dihydroceramide desaturase and dihydrosphingolipids: debutant players in the sphingolipid arena.Prog Lipid Res. 2012 Apr;51(2):82-94. doi: 10.1016/j.plipres.2011.12.002. Epub 2011 Dec 17. Prog Lipid Res. 2012. PMID: 22200621 Review.
Cited by
-
The Complex Tail of Circulating Sphingolipids in Atherosclerosis and Cardiovascular Disease.J Lipid Atheroscler. 2021 Sep;10(3):268-281. doi: 10.12997/jla.2021.10.3.268. Epub 2021 May 6. J Lipid Atheroscler. 2021. PMID: 34621698 Free PMC article. Review.
-
Alterations and correlations of gut microbiota, fecal, and serum metabolome characteristics in a rat model of alcohol use disorder.Front Microbiol. 2023 Jan 4;13:1068825. doi: 10.3389/fmicb.2022.1068825. eCollection 2022. Front Microbiol. 2023. PMID: 36687619 Free PMC article.
-
Dietary glucosylceramide enhances cornified envelope formation via transglutaminase expression and involucrin production.Lipids. 2011 Jun;46(6):529-35. doi: 10.1007/s11745-011-3546-0. Epub 2011 Mar 17. Lipids. 2011. PMID: 21416143
-
Role and interaction of bacterial sphingolipids in human health.Front Microbiol. 2023 Oct 23;14:1289819. doi: 10.3389/fmicb.2023.1289819. eCollection 2023. Front Microbiol. 2023. PMID: 37937219 Free PMC article. Review.
-
Gut symbiont-derived sphingosine modulates vector competence in Aedes mosquitoes.Nat Commun. 2024 Sep 19;15(1):8221. doi: 10.1038/s41467-024-52566-1. Nat Commun. 2024. PMID: 39300135 Free PMC article.
References
-
- Merrill A. H., Jr., Schmelz E. M., Dillehay D. L., Spiegel S., Shayman J. A., Schroeder J. J., Riley R. T., Voss K. A., Wang E. 1997. Sphingolipids–the enigmatic lipid class: biochemistry, physiology, and pathophysiology. Toxicol. Appl. Pharmacol. 142: 208–225. - PubMed
-
- Sawai H., Domae N., Okazaki T. 2005. Current status and perspectives in ceramide-targeting molecular medicine. Curr. Pharm. Des. 11: 2479–2487. - PubMed
-
- Rawlings A. V. 2003. Trends in stratum corneum research and the management of dry skin conditions. Int. J. Cosmet. Sci. 25: 63–95. - PubMed
-
- Imokawa G., Abe A., Jin K., Higaki Y., Kawashima M., Hidano A. 1991. Decreased level of ceramides in stratum corneum of atopic dermatitis: an etiologic factor in atopic dry skin? J. Invest. Dermatol. 96: 523–526. - PubMed
-
- Kim D. W., Park J. Y., Na G. Y., Lee S. J., Lee W. J. 2006. Correlation of clinical features and skin barrier function in adolescent and adult patients with atopic dermatitis. Int. J. Dermatol. 45: 698–701. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

