Distinct cell death programs in monocytes regulate innate responses following challenge with common causes of invasive bacterial disease
- PMID: 20656927
- PMCID: PMC2929480
- DOI: 10.4049/jimmunol.1000805
Distinct cell death programs in monocytes regulate innate responses following challenge with common causes of invasive bacterial disease
Abstract
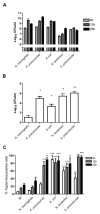
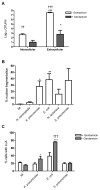
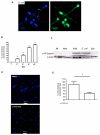
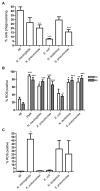
Peripheral blood monocytes represent the rapid response component of mononuclear phagocyte host defense, generating vigorous but finite antibacterial responses. We investigated the fate of highly purified primary human monocytes following phagocytosis of different bacteria. Exposure to high bacterial loads resulted in rapid loss of cell viability and decreased functional competence. Cell death typically involved classical apoptosis. Exposure to high numbers of Escherichia coli and Klebsiella pneumoniae induced nonapoptotic death with loss of cell membrane integrity, marked disruption of phagolysosomes, and caspase-1 activation, while a subset of cells also released caspase-1-regulated extracellular traps. Classical apoptosis increased if extracellular bacterial replication was reduced and decreased if intracellular ATP levels were reduced during these infections. Both classical apoptosis and the alternative forms of cell death allowed monocytes, whose functional competence was exhausted, to downregulate reactive oxygen species and proinflammatory cytokine responses. In contrast, sustained stimulation of glycolytic metabolism and mitochondrial oxidative phosphorylation, with associated hypoxia inducible factor-1alpha upregulation, maintained intracellular ATP levels and prolonged monocyte functional longevity, as assessed by maintenance of phagocytosis, reactive oxygen species production, and proinflammatory cytokine generation. Monocyte innate responses to bacteria are short-lived and are limited by an intrinsic program of apoptosis, a response that is subverted by overwhelming infection with E. coli and K. pneumoniae or bacterial stimulation of cell metabolism. In this regard, the fate of monocytes following bacterial challenge more closely resembles neutrophils than macrophages.
Figures



References
-
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

