Metabolic regulator betaKlotho interacts with fibroblast growth factor receptor 4 (FGFR4) to induce apoptosis and inhibit tumor cell proliferation
- PMID: 20657013
- PMCID: PMC2943257
- DOI: 10.1074/jbc.M110.148288
Metabolic regulator betaKlotho interacts with fibroblast growth factor receptor 4 (FGFR4) to induce apoptosis and inhibit tumor cell proliferation
Abstract
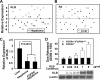
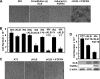
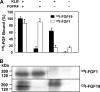
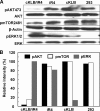
In organs involved in metabolic homeostasis, transmembrane α and βklothos direct FGFR signaling to control of metabolic pathways. Coordinate expression of βklotho and FGFR4 is a property of mature hepatocytes. Genetic deletion of FGFR4 or βklotho in mice disrupts hepatic cholesterol/bile acid and lipid metabolism. The deletion of FGFR4 has no effect on the proliferative response of hepatocytes after liver injury. However, its absence results in accelerated progression of dimethynitrosamine-initiated hepatocellular carcinomas, indicating that FGFR4 suppresses hepatoma proliferation. The mechanism underlying the FGFR4-mediated hepatoma suppression has not been addressed. Here we show that βklotho expression is more consistently down-regulated in human and mouse hepatomas than FGFR4. Co-expression and activation by either endocrine FGF19 or cellular FGF1 of the FGFR4 kinase in a complex with βklotho restricts cell population growth through induction of apoptotic cell death in both hepatic and nonhepatic cells. The βklotho-FGFR4 partnership caused a depression of activated AKT and mammalian target of rapamycin while activating ERK1/2 that may underlie the pro-apoptotic effect. Our results show that βklotho not only interacts with heparan sulfate-FGFR4 to form a complex with high affinity for endocrine FGF19 but also impacts the quality of downstream signaling and biological end points activated by either FGF19 or canonical FGF1. Thus the same βklotho-heparan sulfate-FGFR4 partnership that mediates endocrine control of hepatic metabolism plays a role in cellular homeostasis and hepatoma suppression through negative control of cell population growth mediated by pro-apoptotic signaling.
Figures




References
-
- McKeehan W. L., Wang F., Kan M. (1998) Prog. Nucleic Acid Res. Mol. Biol. 59, 135–176 - PubMed
-
- Eswarakumar V. P., Lax I., Schlessinger J. (2005) Cytokine Growth Factor Rev. 16, 139–149 - PubMed
-
- Luo Y., Ye S., Kan M., McKeehan W. L. (2006) J. Biol. Chem. 281, 21052–21061 - PubMed
-
- Turner N., Grose R. (2010) Nat. Rev. Cancer. 10, 116–129 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

