Female X-chromosome mosaicism for gp91phox expression diversifies leukocyte responses during endotoxemia
- PMID: 20657276
- PMCID: PMC3045076
- DOI: 10.1097/CCM.0b013e3181eb9ed6
Female X-chromosome mosaicism for gp91phox expression diversifies leukocyte responses during endotoxemia
Abstract
Objective: To test the hypothesis, using an animal model, whether female X-chromosome mosaicism for inflammatory gene expression could contribute to the gender dimorphic response during the host response. X-chromosome-linked genetic polymorphisms present a unique biological condition because females display heterozygous cellular mosaicism, due to the fact that either the maternal or the paternal X chromosomes are inactivated in each individual cell in females. This is in contrast with the conditions in males who carry exclusively the maternal X chromosome.
Design: Prospective, randomized, laboratory investigation.
Settings: University research laboratory.
Subjects: Female mice deficient, heterozygous (mosaic) or WT for the X-linked gp91phox.
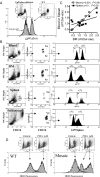
Interventions: We compared selected inflammatory markers among heterozygous (mosaics), WT and homozygous deficient animals in response to in vivo lipopolysaccharide (Escherichia coli, 20 mg/kg body weight). To test individual mosaic subpopulations of polymorphonuclear neutrophil responses, we also developed a flow cytometry assay that identifies the active parental X chromosomes in individual cells, using gp91phox expression as a marker.
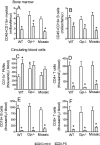
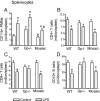
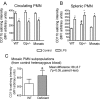
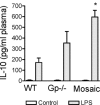
Measurements and main results: Heterozygous mosaic mice presented white blood cell trafficking patterns similar to that observed in WT mice, despite the fact that the deficient subpopulation in mosaic animals displayed increased cell activation as reflected in elevated neutrophil CD11b expression and splenic infiltration. Mosaic animals also displayed splenic neutrophil infiltration, which was skewed toward the deficient subpopulation. Observations on splenic T-cell depletion and post lipopolysaccharide interleukin-10 responses indicated that the inflammatory response in mosaic animals does not simply display an average of the deficient and WT responses, but the mosaic subjects display a uniquely characteristic response.
Conclusions: The study supports the notion that female X chromosome mosaicism for polymorphic gene expression represents a unique condition, which may contribute to the gender dimorphic character of the inflammatory response. Mosaicism for X-linked polymorphisms may have clinical significance and needs consideration in genetic association or gender-related clinical studies.
Figures

Similar articles
-
Female X-chromosome mosaicism for NOX2 deficiency presents unique inflammatory phenotype and improves outcome in polymicrobial sepsis.J Immunol. 2011 Jun 1;186(11):6465-73. doi: 10.4049/jimmunol.1100205. Epub 2011 Apr 18. J Immunol. 2011. PMID: 21502376 Free PMC article.
-
Cellular mosaicism for X-linked polymorphisms and IRAK1 expression presents a distinct phenotype and improves survival following sepsis.J Leukoc Biol. 2014 Mar;95(3):497-507. doi: 10.1189/jlb.0713397. Epub 2013 Nov 5. J Leukoc Biol. 2014. PMID: 24193737 Free PMC article.
-
X-Linked IRAK1 Polymorphism is Associated with Sex-Related Differences in Polymorphonuclear Granulocyte and Monocyte Activation and Response Variabilities.Shock. 2020 Apr;53(4):434-441. doi: 10.1097/SHK.0000000000001404. Shock. 2020. PMID: 31306349 Free PMC article.
-
The X-files of inflammation: cellular mosaicism of X-linked polymorphic genes and the female advantage in the host response to injury and infection.Shock. 2007 Jun;27(6):597-604. doi: 10.1097/SHK.0b013e31802e40bd. Shock. 2007. PMID: 17505297 Review.
-
Mosaics and haemophilia.Haemophilia. 2009 Nov;15(6):1181-6. doi: 10.1111/j.1365-2516.2009.02003.x. Epub 2009 Mar 11. Haemophilia. 2009. PMID: 19473426 Review.
Cited by
-
Inherent X-Linked Genetic Variability and Cellular Mosaicism Unique to Females Contribute to Sex-Related Differences in the Innate Immune Response.Front Immunol. 2017 Nov 13;8:1455. doi: 10.3389/fimmu.2017.01455. eCollection 2017. Front Immunol. 2017. PMID: 29180997 Free PMC article.
-
Sex Differences in Case Fatality Rate of COVID-19: Insights From a Multinational Registry.Mayo Clin Proc. 2020 Aug;95(8):1613-1620. doi: 10.1016/j.mayocp.2020.05.014. Epub 2020 May 29. Mayo Clin Proc. 2020. PMID: 32753136 Free PMC article.
-
Gender differences in sepsis: cardiovascular and immunological aspects.Virulence. 2014 Jan 1;5(1):12-9. doi: 10.4161/viru.26982. Epub 2013 Nov 5. Virulence. 2014. PMID: 24193307 Free PMC article. Review.
-
Bimodal role of NADPH oxidases in the regulation of biglycan-triggered IL-1β synthesis.Matrix Biol. 2016 Jan;49:61-81. doi: 10.1016/j.matbio.2015.12.005. Epub 2015 Dec 12. Matrix Biol. 2016. PMID: 26689330 Free PMC article.
-
Female X-chromosome mosaicism for NOX2 deficiency presents unique inflammatory phenotype and improves outcome in polymicrobial sepsis.J Immunol. 2011 Jun 1;186(11):6465-73. doi: 10.4049/jimmunol.1100205. Epub 2011 Apr 18. J Immunol. 2011. PMID: 21502376 Free PMC article.
References
-
- Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocr Rev. 2003;24:313–340. - PubMed
-
- Chen CC, Parker CR., Jr Adrenal androgens and the immune system. Semin Reprod Med. 2004;22:369–377. - PubMed
-
- Angele MK, Schwacha MG, Ayala A, et al. Effect of gender and sex hormones on immune responses following shock. Shock. 2000;14:81–90. - PubMed
-
- Kovacs EJ, Messingham KA, Gregory MS. Estrogen regulation of immune responses after injury. Mol Cell Endocrinol. 2002;193:129–135. - PubMed
-
- Choudhry MA, Bland KI, Chaudry IH. Gender and susceptibility to sepsis following trauma. Endocr Metab Immune Disord Drug Targets. 2006;6:127–135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

