Bovine and human serum albumin interactions with 3-carboxyphenoxathiin studied by fluorescence and circular dichroism spectroscopy
- PMID: 20657416
- PMCID: PMC6257641
- DOI: 10.3390/molecules15063905
Bovine and human serum albumin interactions with 3-carboxyphenoxathiin studied by fluorescence and circular dichroism spectroscopy
Abstract
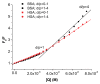
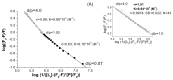
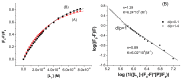
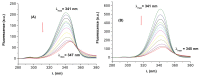
The interactions of 3-carboxyphenoxathiin with Bovine Serum Albumin (BSA) and Human Serum Albumin (HSA) have been studied by fluorescence and circular dichroism spectroscopy. The binding of 3-carboxyphenoxathiin quenches the BSA and HSA fluorescence, revealing a 1:1 interaction with a binding constant of about 10(5) M(-1). In addition, according to the synchronous fluorescence spectra of BSA and HSA in presence of 3-carboxyphenoxathiin, the tryptophan residues of the proteins are most perturbed by the binding process. Finally, the distance between the acceptor, 3-carboxyphenoxathiin, and the donor, BSA or HSA, was estimated on the basis of the Förster resonance energy transfer (FRET). The fluorescence results are correlated with those obtained from the circular dichroism spectra, which reveal the change of the albumin conformation during the interaction process.
Figures




Similar articles
-
Spectroscopic studies on the interaction of bovine (BSA) and human (HSA) serum albumins with ionic surfactants.Spectrochim Acta A Mol Biomol Spectrosc. 2000 Oct;56A(11):2255-71. doi: 10.1016/s1386-1425(00)00313-9. Spectrochim Acta A Mol Biomol Spectrosc. 2000. PMID: 11058071
-
Analysis of binding interaction of curcumin and diacetylcurcumin with human and bovine serum albumin using fluorescence and circular dichroism spectroscopy.Protein J. 2009 May;28(3-4):189-96. doi: 10.1007/s10930-009-9184-1. Protein J. 2009. PMID: 19495944
-
Study on the interaction of Co (III) DiAmsar with serum albumins: spectroscopic and molecular docking methods.Spectrochim Acta A Mol Biomol Spectrosc. 2015 Jan 25;135:410-6. doi: 10.1016/j.saa.2014.06.078. Epub 2014 Jul 4. Spectrochim Acta A Mol Biomol Spectrosc. 2015. PMID: 25105263
-
Study on the interactions of mapenterol with serum albumins using multi-spectroscopy and molecular docking.Luminescence. 2016 Mar;31(2):372-379. doi: 10.1002/bio.2969. Epub 2015 Jul 14. Luminescence. 2016. PMID: 26179292
-
Study of the interaction of an anticancer drug with human and bovine serum albumin: spectroscopic approach.J Pharm Biomed Anal. 2006 May 3;41(2):393-9. doi: 10.1016/j.jpba.2005.11.037. Epub 2006 Jan 18. J Pharm Biomed Anal. 2006. PMID: 16413740
Cited by
-
Synthesis and Structural Investigation of New Bio-Relevant Complexes of Lanthanides with 5-Hydroxyflavone: DNA Binding and Protein Interaction Studies.Molecules. 2016 Dec 16;21(12):1737. doi: 10.3390/molecules21121737. Molecules. 2016. PMID: 27999283 Free PMC article.
-
A comparative analysis on the binding characteristics of various mammalian albumins towards a multitherapeutic agent, pinostrobin.Exp Anim. 2015;64(2):101-8. doi: 10.1538/expanim.14-0053. Epub 2014 Dec 16. Exp Anim. 2015. PMID: 25519455 Free PMC article.
-
Interaction of Di-2-pyridylketone 2-pyridine Carboxylic Acid Hydrazone and Its Copper Complex with BSA: Effect on Antitumor Activity as Revealed by Spectroscopic Studies.Molecules. 2016 Apr 28;21(5):563. doi: 10.3390/molecules21050563. Molecules. 2016. PMID: 27136517 Free PMC article.
-
Binding of an anti-inflammatory drug lornoxicam with blood proteins: insights from spectroscopic investigations.J Fluoresc. 2011 Mar;21(2):487-95. doi: 10.1007/s10895-010-0735-9. Epub 2010 Oct 6. J Fluoresc. 2011. PMID: 20924657
-
Mass Spectrometric and Spectrofluorometric Studies of the Interaction of Aristolochic Acids with Proteins.Sci Rep. 2015 Oct 16;5:15192. doi: 10.1038/srep15192. Sci Rep. 2015. PMID: 26471474 Free PMC article.
References
-
- Jones L.J., Haugland R.P., Singer V.L. Development and characterization of the NanoOrange protein quantitation assay: A fluorescence-based assay of proteins in solution. Biotechniques. 2003;34:850–854. - PubMed
-
- Ionescu S., Gavriliu D., Maior O., Hillebrand M. Excited states properties of some phenoxathiin derivatives. J. Photochem. Photobiol. 1999;124:67–73. doi: 10.1016/S1010-6030(99)00058-1. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

