Bovine and human serum albumin interactions with 3-carboxyphenoxathiin studied by fluorescence and circular dichroism spectroscopy
- PMID: 20657416
- PMCID: PMC6257641
- DOI: 10.3390/molecules15063905
Bovine and human serum albumin interactions with 3-carboxyphenoxathiin studied by fluorescence and circular dichroism spectroscopy
Abstract
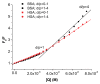
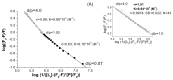
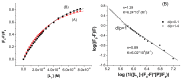
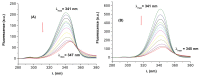
The interactions of 3-carboxyphenoxathiin with Bovine Serum Albumin (BSA) and Human Serum Albumin (HSA) have been studied by fluorescence and circular dichroism spectroscopy. The binding of 3-carboxyphenoxathiin quenches the BSA and HSA fluorescence, revealing a 1:1 interaction with a binding constant of about 10(5) M(-1). In addition, according to the synchronous fluorescence spectra of BSA and HSA in presence of 3-carboxyphenoxathiin, the tryptophan residues of the proteins are most perturbed by the binding process. Finally, the distance between the acceptor, 3-carboxyphenoxathiin, and the donor, BSA or HSA, was estimated on the basis of the Förster resonance energy transfer (FRET). The fluorescence results are correlated with those obtained from the circular dichroism spectra, which reveal the change of the albumin conformation during the interaction process.
Figures




References
-
- Jones L.J., Haugland R.P., Singer V.L. Development and characterization of the NanoOrange protein quantitation assay: A fluorescence-based assay of proteins in solution. Biotechniques. 2003;34:850–854. - PubMed
-
- Ionescu S., Gavriliu D., Maior O., Hillebrand M. Excited states properties of some phenoxathiin derivatives. J. Photochem. Photobiol. 1999;124:67–73. doi: 10.1016/S1010-6030(99)00058-1. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

