Synthesis and biological evaluation of a gamma-cyclodextrin-based formulation of the anticancer agent 5,6,11,12,17,18,23,24-octahydrocyclododeca[1,2-b:4,5-b':7,8-b'':10,11-b''']tetraindole (CTet)
- PMID: 20657428
- PMCID: PMC6264452
- DOI: 10.3390/molecules15064085
Synthesis and biological evaluation of a gamma-cyclodextrin-based formulation of the anticancer agent 5,6,11,12,17,18,23,24-octahydrocyclododeca[1,2-b:4,5-b':7,8-b'':10,11-b''']tetraindole (CTet)
Abstract
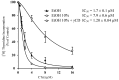
5,6,11,12,17,18,23,24-Octahydrocyclododeca[1,2-b:4,5-b':7,8-b'':10,11- b''']tetrai ndole (CTet), an indole-3-carbinol (I3C) metabolite endowed with anticancer properties, is poorly soluble in the solvents most frequently used in biological tests. This study indicates that the use of gamma-cyclodextrin (gamma-CD) avoids this problem. Formulated with gamma-CD CTet is a potent inhibitor of DNA synthesis in both estrogen receptor positive (MCF-7) and estrogen receptor negative (MDA-MB-231) human breast cell lines (IC50 = 1.20 +/- 0.04 microM and 1.0 +/- 0.1 microM, respectively).
Figures


References
-
- Peter F.M., editor. National Research Council. Diet, Nutrition and Cancer. National Academy Press; Washington, DC, USA: 1982. pp. 358–370.
-
- Wattenberg L.W., Loub W.D. Inhibition of polycyclic aromatic hydrocarbon-induced neoplasia by naturally occurring indoles. Cancer Res. 1978;38:1410–1413. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

