Construction of eight-membered carbocycles with trisubstituted double bonds using the ring closing metathesis reaction
- PMID: 20657438
- PMCID: PMC6264272
- DOI: 10.3390/molecules15064242
Construction of eight-membered carbocycles with trisubstituted double bonds using the ring closing metathesis reaction
Abstract
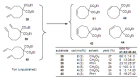
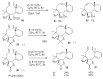
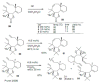
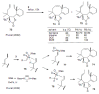
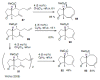
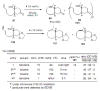
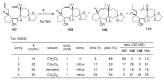
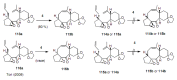
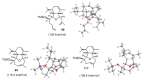
Medium sized carbocycles are particularly difficult to synthesize. Ring closing metathesis reactions (RCM) have recently been applied to construct eight-membered carbocycles, but trisubstituted double bonds in the eight-membered rings are more difficult to produce using RCM reactions. In this review, model examples and our own results are cited and the importance of the preparation of suitably designed precursors is discussed. Examples of RCM reactions used in the total synthesis of natural products are also outlined.
Figures
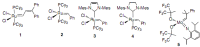
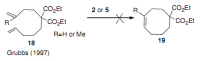

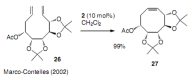
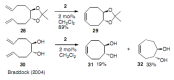
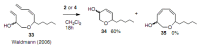
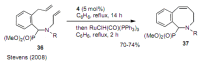
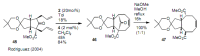
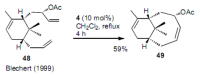
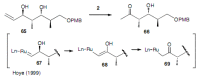
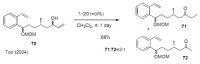
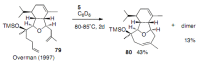
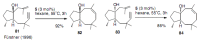
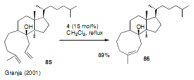
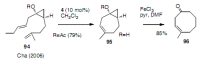
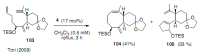
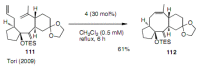

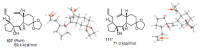
Similar articles
-
Use of RCM reactions for construction of eight-membered carbocycles and introduction of a hydroxy group at the juncture between five- and eight-membered carbocycles.Nat Prod Commun. 2013 Jul;8(7):883-7. Nat Prod Commun. 2013. PMID: 23980416
-
Recent Advances in the Total Synthesis of Natural Products Containing Eight-Membered Carbocycles (2009-2019).Chem Rev. 2020 Jul 8;120(13):5910-5953. doi: 10.1021/acs.chemrev.0c00045. Epub 2020 Apr 28. Chem Rev. 2020. PMID: 32343125 Review.
-
Intramolecular silicon-assisted cross-coupling reactions: general synthesis of medium-sized rings containing a 1,3-cis-cis diene unit.J Am Chem Soc. 2002 Mar 13;124(10):2102-3. doi: 10.1021/ja0178158. J Am Chem Soc. 2002. PMID: 11878949
-
Ring-closing metathesis as a basis for the construction of aromatic compounds.Angew Chem Int Ed Engl. 2006 Apr 21;45(17):2664-70. doi: 10.1002/anie.200503512. Angew Chem Int Ed Engl. 2006. PMID: 16548035 Review.
-
A palladium/chiral amine co-catalyzed enantioselective dynamic cascade reaction: synthesis of polysubstituted carbocycles with a quaternary carbon stereocenter.Angew Chem Int Ed Engl. 2013 Jun 3;52(23):6050-4. doi: 10.1002/anie.201300559. Epub 2013 Apr 25. Angew Chem Int Ed Engl. 2013. PMID: 23620447
Cited by
-
Evolution of a Unified Strategy for Complex Sesterterpenoids: Progress toward Astellatol and the Total Synthesis of (-)-Nitidasin.Chemistry. 2015 Sep 21;21(39):13646-65. doi: 10.1002/chem.201501423. Epub 2015 Aug 20. Chemistry. 2015. PMID: 26300211 Free PMC article.
-
Synthesis of indole-based propellane derivatives via Weiss-Cook condensation, Fischer indole cyclization, and ring-closing metathesis as key steps.Beilstein J Org Chem. 2013 Nov 29;9:2709-14. doi: 10.3762/bjoc.9.307. eCollection 2013. Beilstein J Org Chem. 2013. PMID: 24367436 Free PMC article.
-
Kinetic resolution of 1-(1-alkynyl)cyclopropyl ketones via gold-catalyzed divergent (4 + 4) cycloadditions: stereoselective access to furan fused eight-membered heterocycles.Chem Sci. 2024 May 22;15(24):9361-9368. doi: 10.1039/d4sc02763a. eCollection 2024 Jun 19. Chem Sci. 2024. PMID: 38903218 Free PMC article.
References
-
- Nguyen S.T., Johnson L.K., Grubbs R.H. Ring-opening metathesis polymerization (ROMP) of norbornene by a group VIII carbene complex in protic media. J. Am. Chem. Soc. 1992;114:3974–3975.
-
- Nguyen S.T., Grubbs R.H. Syntheses and activities of new single-component, ruthenium-based olefin metathesis catalysis. J. Am. Chem. Soc. 1993;115:9858–9859.
-
- Grubbs R.H., Miller S.J., Fu G.C. Ring-closing metathesis and related processes in organic synthesis. Acc. Chem. Res. 1995;28:446–452. doi: 10.1021/ar00059a002. - DOI
-
- Schwab P., France M.B., Ziller J.W., Grubbs R.H. A series of well-defined metathesis catalysts-synthesis of [RuCl2(CHR)(PR3)2] and its reactions. Angew. Chem. Int. Ed. Engl. 1995;34:2039–2041. doi: 10.1002/anie.199520391. - DOI
-
- Schwab P., Grubbs R.H., Ziller J.W. Synthesis and applications of RuCl2(=CHR’)(PR3)2: The influence of the alkylidene moiety on metathesis activity. J. Am. Chem. Soc. 1996;118:100–110.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

