Artificial self-sufficient P450 in reversed micelles
- PMID: 20657456
- PMCID: PMC6257473
- DOI: 10.3390/molecules15052935
Artificial self-sufficient P450 in reversed micelles
Abstract
Cytochrome P450s are heme-containing monooxygenases that require electron transfer proteins for their catalytic activities. They prefer hydrophobic compounds as substrates and it is, therefore, desirable to perform their reactions in non-aqueous media. Reversed micelles can stably encapsulate proteins in nano-scaled water pools in organic solvents. However, in the reversed micellar system, when multiple proteins are involved in a reaction they can be separated into different micelles and it is then difficult to transfer electrons between proteins. We show here that an artificial self-sufficient cytochrome P450, which is an enzymatically crosslinked fusion protein composed of P450 and electron transfer proteins, showed micelle-size dependent catalytic activity in a reversed micellar system. Furthermore, the presence of thermostable alcohol dehydrogenase promoted the P450-catalyzed reaction due to cofactor regeneration.
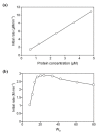
Figures








References
-
- Bell G., Halling P.J., Moore B.D., Partridge J., Rees D.G. Biocatalyst Behaviour in Low-water Systems. Trends Biotechnol. 1995;13:468–473. doi: 10.1016/S0167-7799(00)89004-6. - DOI
-
- Eckstein M., Dauβmann T., Kragl U. Recent Development in NAD(P)H Regeneration for Enzymatic Reductions in One- and Two-Phase Systems. Biocatal. Biotrans. 2004;22:89–96. doi: 10.1080/10242420410001692769. - DOI
-
- Klyachko N.L., Levashov A.V. Bioorganic Synthesis in Reverse Micelles and Related Systems. Curr. Opin. Colloid Interf. Dci. 2003;8:179–186. doi: 10.1016/S1359-0294(03)00016-5. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

