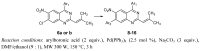Regioselective Suzuki-Miyaura reaction: application to the microwave-promoted synthesis of 4,7-diarylquinazolines
- PMID: 20657457
- PMCID: PMC6263357
- DOI: 10.3390/molecules15052949
Regioselective Suzuki-Miyaura reaction: application to the microwave-promoted synthesis of 4,7-diarylquinazolines
Abstract
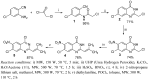
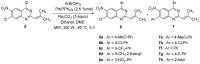
New diarylquinazolines displaying pharmaceutical potential were synthesized in high yields from 4,7-dichloro-2-(2-methylprop-1-enyl)-6-nitroquinazoline by using microwave-promoted regioselective Suzuki-Miyaura cross-coupling reactions.
Figures
Similar articles
-
Synthesis, characterization and microwave-promoted catalytic activity of novel N-phenylbenzimidazolium salts in Heck-Mizoroki and Suzuki-Miyaura cross-coupling reactions under mild conditions.Molecules. 2013 Feb 25;18(3):2501-17. doi: 10.3390/molecules18032501. Molecules. 2013. PMID: 23439565 Free PMC article.
-
Facile synthesis of 6-aryl 5-N-substituted pyridazinones: microwave-assisted Suzuki-Miyaura cross coupling of 6-chloropyridazinones.J Org Chem. 2008 Sep 19;73(18):7204-8. doi: 10.1021/jo801097v. Epub 2008 Aug 8. J Org Chem. 2008. PMID: 18687004
-
Microwave-assisted reactions in heterocyclic compounds with applications in medicinal and supramolecular chemistry.Comb Chem High Throughput Screen. 2007 Dec;10(10):877-902. doi: 10.2174/138620707783220347. Comb Chem High Throughput Screen. 2007. PMID: 18288949 Review.
-
Regiocontrolled microwave assisted bifunctionalization of 7,8-dihalogenated imidazo[1,2-a]pyridines: a one pot double-coupling approach.Molecules. 2012 Sep 6;17(9):10683-707. doi: 10.3390/molecules170910683. Molecules. 2012. PMID: 22955457 Free PMC article.
-
Development of green methodologies for Heck, Chan-Lam, Stille and Suzuki cross-coupling reactions.Mol Divers. 2020 Aug;24(3):821-839. doi: 10.1007/s11030-019-09988-7. Epub 2019 Aug 28. Mol Divers. 2020. PMID: 31463833 Review.
Cited by
-
Site-selective Suzuki-Miyaura coupling of heteroaryl halides - understanding the trends for pharmaceutically important classes.Chem Sci. 2017 Jan 1;8(1):40-62. doi: 10.1039/c6sc02118b. Epub 2016 Aug 9. Chem Sci. 2017. PMID: 28451148 Free PMC article.
-
Synthesis and promising in vitro antiproliferative activity of sulfones of a 5-nitrothiazole series.Molecules. 2012 Dec 21;18(1):97-113. doi: 10.3390/molecules18010097. Molecules. 2012. PMID: 23344190 Free PMC article.
-
8-Aryl-6-chloro-3-nitro-2-(phenylsulfonylmethyl)imidazo[1,2-a]pyridines as potent antitrypanosomatid molecules bioactivated by type 1 nitroreductases.Eur J Med Chem. 2018 Sep 5;157:115-126. doi: 10.1016/j.ejmech.2018.07.064. Epub 2018 Aug 1. Eur J Med Chem. 2018. PMID: 30092366 Free PMC article.
-
Advances in metal-catalyzed cross-coupling reactions of halogenated quinazolinones and their quinazoline derivatives.Molecules. 2014 Oct 29;19(11):17435-63. doi: 10.3390/molecules191117435. Molecules. 2014. PMID: 25356566 Free PMC article. Review.
References
-
- Miyaura N., Yamada K., Suzuki A. A new stereospecific cross-coupling by the palladium-catalyzed reaction of 1-alkenylboranes with 1-alkenyl or 1-alkynyl halides. Tetrahedron Lett. 1979;20:3437–3440. doi: 10.1016/S0040-4039(01)95429-2. - DOI
-
- Miyaura N., Suzuki A. Stereoselective synthesis of arylated (E)-alkenes by the reaction of alk-1-enylboranes with aryl halides in the presence of palladium catalyst. J. Chem. Soc. Chem. Commun. 1979:866–867. doi: 10.1039/c39790000866. - DOI
-
- Miyaura N., Suzuki A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995;95:2457–2483. doi: 10.1021/cr00039a007. - DOI
-
- Miyaura N. Organoboron Compounds. Top. Curr. Chem. 2002;219:11–59. doi: 10.1007/3-540-45313-X_2. - DOI
-
- Suzuki A., Brown H.C. Organic Syntheses via BoranesVolume 3: Suzuki coupling. Aldrich Chemical Company; Milwaukee, WI, USA: 2003.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources