Bohlmann-Rahtz cyclodehydration of aminodienones to pyridines using N-iodosuccinimide
- PMID: 20657473
- PMCID: PMC6263277
- DOI: 10.3390/molecules15053211
Bohlmann-Rahtz cyclodehydration of aminodienones to pyridines using N-iodosuccinimide
Abstract
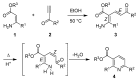
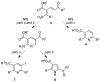
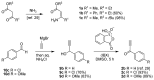
Cyclodehydration of Bohlmann-Rahtz aminodienone intermediates using N-iodosuccinimide as a Lewis acid proceeds at low temperature under very mild conditions to give the corresponding 2,3,6-trisubstituted pyridines in high yield and with total regiocontrol.
Figures

References
-
- Roth H.J., Kleemann A. Pharmaceutical Chemistry. Drug Synthesis. Vol. 1 John Wiley & Sons; New York, NY, USA: 1988.
-
- Henkel T., Brunne R.M., Müller H., Reichel F. Statistical investigation into the structural complexity of natural products and synthetic compounds. Angew. Chem. Int. Ed. Engl. 1999;38:643–647. - PubMed
-
- Hantzsch A. Condensationprodukte aus Aldehydammoniak und Ketoniartigen Verbindungen. Berichte. 1881;14:1637–1638.
-
- Bohlmann F., Rahtz D. Űber eine neue pyridinsynthese. Chem. Ber. 1957;90:2265–2272. doi: 10.1002/cber.19570901021. - DOI
-
- Bagley M.C., Glover C., Merritt E.A. The Bohlmann–Rahtz pyridine synthesis: from discovery to applications. Synlett. 2007:2459–2482.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

