Vitamin B12: unique metalorganic compounds and the most complex vitamins
- PMID: 20657474
- PMCID: PMC6257451
- DOI: 10.3390/molecules15053228
Vitamin B12: unique metalorganic compounds and the most complex vitamins
Abstract
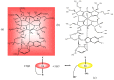
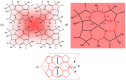
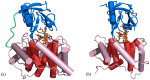
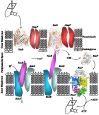
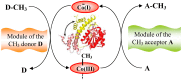
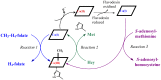
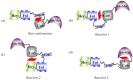
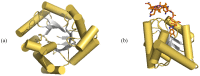
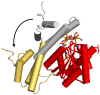
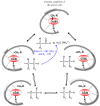
The chemistry and biochemistry of the vitamin B(12) compounds (cobalamins, XCbl) are described, with particular emphasis on their structural aspects and their relationships with properties and function. A brief history of B(12), reveals how much the effort of chemists, biochemists and crystallographers have contributed in the past to understand the basic properties of this very complex vitamin. The properties of the two cobalamins, the two important B(12) cofactors Ado- and MeCbl are described, with particular emphasis on how the Co-C bond cleavage is involved in the enzymatic mechanisms. The main structural features of cobalamins are described, with particular reference to the axial fragment. The structure/property relationships in cobalamins are summarized. The recent studies on base-off/base-on equilibrium are emphasized for their relevance to the mode of binding of the cofactor to the protein scaffold. The absorption, transport and cellular uptake of cobalamins and the structure of the B(12) transport proteins, IF and TC, in mammals are reviewed. The B(12) transport in bacteria and the structure of the so far determined proteins are briefly described. The currently accepted mechanisms for the catalytic cycles of the AdoCbl and MeCbl enzymes are reported. The structure and function of B(12) enzymes, particularly the important mammalian enzymes methyltransferase (MetH) and methyl-malonyl-coenzyme A mutase (MMCM), are described and briefly discussed. Since fast proliferating cells require higher amount of vitamin B(12) than that required by normal cells, the study of B(12 )conjugates as targeting agents has recently gained importance. Bioconjugates have been studied as potential agents for delivering radioisotopes and NMR probes or as various cytotoxic agents towards cancer cells in humans and the most recent studies are described. Specifically, functionalized bioconjugates are used as "Trojan horses" to carry into the cell the appropriate antitumour or diagnostic label. Possible future developments of B(12) work are summarized.
Figures






References
-
- Folkers K. History of Vitamin B12: Pernicious Anemia to Crystalline Cyanocobalamin. In: Dolphin D., editor. Vitamin B12. Volume I. John Wiley & Sons; New York, NY, USA: 1982. pp. 1–15.
-
- Hodgkin D.C. New and Old Problem in the Structure Analysis of Vitamin B12. In: Zagalak B., Friedrich W., editors. Vitamin B12, Proceedings of the Third European Symposium on Vitamin B12 and Intrinsic Factors, Zurich, Switzerland, March 1999. Walter de Gruyter; Berlin, Germany: 1979. pp. 19–36.
-
- Woodward R.B. Synthetic vitamin B12. In: Zagalak B., Friedrich W., editors. Vitamin B12, Proceedings of the Third European Symposium on Vitamin B12 and Intrinsic Factors, Zurich, Switzerland, March 1999. Walter de Gruyter; Berlin: Germany; 1979. pp. 37–88.
-
- Kräutler B. In: Vitamin B12 and B12 Proteins. Kräutler B., Arigoni D., Golding B.T., editors. Wiley-VCH; Weinheim, Germany: 1998. pp. 3–43.
-
- Hogenkamp H.P.C. In: Chemistry and Biochemistry of B12. Banerjee R., editor. John Wiley & Sons; New York, NY, USA: 1999. pp. 3–8.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

