Microwave synthesis, basic spectral and biological evaluation of some copper (II) mesoporphyrinic complexes
- PMID: 20657510
- PMCID: PMC6263333
- DOI: 10.3390/molecules15053731
Microwave synthesis, basic spectral and biological evaluation of some copper (II) mesoporphyrinic complexes
Abstract
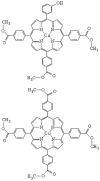
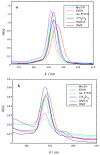
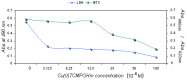
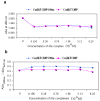
Cu(II) complexes with asymmetrical and symmetrical porphyrinic ligands were synthesized with superior yields using microwave irradiation. The paper presents the synthesis of 5-(3-hydroxyphenyl)-10,15,20-tris-(4-carboxymethylphenyl)-21,23-Cu(II)-porphine in comparison to its symmetrical complex 5,10,15,20-meso-tetrakis-(4-carboxy-methylphenyl)-21,23-Cu(II) porphine. The two compounds were characterized by FT-IR, UV-Vis and EPR spectroscopy, which fully confirmed the structures. The spectral molecular absorption properties of the porphyrinic complexes were studied in organic solvents (methanol, ethanol, iso-propanol, dimethyl sulfoxide, dimethylformamide and methylene chloride), and the influence of the solvent polarity on the absorbance maxima is described. In order to establish their future potential in biomedical applications preliminary toxicological studies consisting of viability and proliferation of standard tumor cell lines (MCF7 and B16) testing was performed. The obtained results indicate a low toxicity for both compounds and further recommends them for testing in light activation protocols.
Figures

References
-
- Moan J., Peng Q. An outline of history of PDT. In: Patrice T., editor. Photodynamic Therapy. Royal Society of Chemistry; Cambridge, UK: 2004. pp. 1–18.
-
- Chen J.Y., Mak N.K., Yow C.M.N., Fung M.C., Chiu L.C., Leung W.N., Cheung N.H. The Binding Characteristics and Intracellular Localization of Temoporfin (mTHPC) in Myeloid Leukemia Cells: Phototoxicity and Mitochondrial Damage. Photochem. Photobiol. 2000;72:541–547. doi: 10.1562/0031-8655(2000)072<0541:TBCAIL>2.0.CO;2. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

