Matriptase initiates activation of epidermal pro-kallikrein and disease onset in a mouse model of Netherton syndrome
- PMID: 20657595
- PMCID: PMC3081165
- DOI: 10.1038/ng.629
Matriptase initiates activation of epidermal pro-kallikrein and disease onset in a mouse model of Netherton syndrome
Abstract
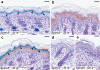
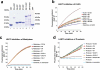
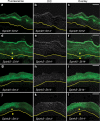
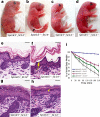
Deficiency in the serine protease inhibitor LEKTI is the etiological origin of Netherton syndrome, which causes detachment of the stratum corneum and chronic inflammation. Here we show that the membrane protease matriptase initiates Netherton syndrome in a LEKTI-deficient mouse model by premature activation of a pro-kallikrein cascade. Auto-activation of pro-inflammatory pro-kallikrein-related peptidases that are associated with stratum corneum detachment was either low or undetectable, but they were efficiently activated by matriptase. Ablation of matriptase from LEKTI-deficient mice dampened inflammation, eliminated aberrant protease activity, prevented detachment of the stratum corneum, and improved the barrier function of the epidermis. These results uncover a pathogenic matriptase-pro-kallikrein pathway that could operate in several human skin and inflammatory diseases.
Figures


Similar articles
-
SPINK5 knockdown in organotypic human skin culture as a model system for Netherton syndrome: effect of genetic inhibition of serine proteases kallikrein 5 and kallikrein 7.Exp Dermatol. 2014 Jul;23(7):524-6. doi: 10.1111/exd.12451. Exp Dermatol. 2014. PMID: 24848304
-
Spink5-deficient mice mimic Netherton syndrome through degradation of desmoglein 1 by epidermal protease hyperactivity.Nat Genet. 2005 Jan;37(1):56-65. doi: 10.1038/ng1493. Epub 2004 Dec 26. Nat Genet. 2005. PMID: 15619623
-
Proteolytic activation cascade of the Netherton syndrome-defective protein, LEKTI, in the epidermis: implications for skin homeostasis.J Invest Dermatol. 2011 Nov;131(11):2223-32. doi: 10.1038/jid.2011.174. Epub 2011 Jun 23. J Invest Dermatol. 2011. PMID: 21697885
-
Physiological and pathological roles of kallikrein-related peptidases in the epidermis.J Dermatol Sci. 2019 Aug;95(2):50-55. doi: 10.1016/j.jdermsci.2019.06.007. Epub 2019 Jun 27. J Dermatol Sci. 2019. PMID: 31279501 Review.
-
Netherton syndrome: defective kallikrein inhibition in the skin leads to skin inflammation and allergy.Biol Chem. 2014 Sep;395(9):945-58. doi: 10.1515/hsz-2014-0137. Biol Chem. 2014. PMID: 25153381 Review.
Cited by
-
Detection of active matriptase using a biotinylated chloromethyl ketone peptide.PLoS One. 2013 Oct 18;8(10):e77146. doi: 10.1371/journal.pone.0077146. eCollection 2013. PLoS One. 2013. PMID: 24204759 Free PMC article.
-
Suppression of Tumorigenicity-14, encoding matriptase, is a critical suppressor of colitis and colitis-associated colon carcinogenesis.Oncogene. 2012 Aug 9;31(32):3679-95. doi: 10.1038/onc.2011.545. Epub 2011 Dec 5. Oncogene. 2012. PMID: 22139080 Free PMC article.
-
Retrospective pharmacogenetic study of psoriasis highlights the role of KLK7 in tumour necrosis factor signalling.Br J Dermatol. 2023 Dec 20;190(1):70-79. doi: 10.1093/bjd/ljad332. Br J Dermatol. 2023. PMID: 37672660 Free PMC article.
-
Hepatocyte growth factor activator inhibitor type 1 maintains the assembly of keratin into desmosomes in keratinocytes by regulating protease-activated receptor 2-dependent p38 signaling.Am J Pathol. 2015 Jun;185(6):1610-23. doi: 10.1016/j.ajpath.2015.02.009. Epub 2015 Apr 1. Am J Pathol. 2015. PMID: 25842366 Free PMC article.
-
Unleashing the therapeutic potential of human kallikrein-related serine proteases.Nat Rev Drug Discov. 2015 Mar;14(3):183-202. doi: 10.1038/nrd4534. Epub 2015 Feb 20. Nat Rev Drug Discov. 2015. PMID: 25698643 Review.
References
-
- Caubet C, et al. Degradation of corneodesmosome proteins by two serine proteases of the kallikrein family, SCTE/KLK5/hK5 and SCCE/KLK7/hK7. J Invest Dermatol. 2004;122:1235–44. - PubMed
-
- Brattsand M, Egelrud T. Purification, molecular cloning, and expression of a human stratum corneum trypsin-like serine protease with possible function in desquamation. J Biol Chem. 1999;274:30033–40. - PubMed
-
- Hansson L, et al. Cloning, expression, and characterization of stratum corneum chymotryptic enzyme. A skin-specific human serine proteinase. J Biol Chem. 1994;269:19420–6. - PubMed
-
- Descargues P, et al. Spink5-deficient mice mimic Netherton syndrome through degradation of desmoglein 1 by epidermal protease hyperactivity. Nat Genet. 2005;37:56–65. - PubMed
-
- Hewett DR, et al. Lethal, neonatal ichthyosis with increased proteolytic processing of filaggrin in a mouse model of Netherton syndrome. Hum Mol Genet. 2005;14:335–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

