MMP-9, uPAR and cathepsin B silencing downregulate integrins in human glioma xenograft cells in vitro and in vivo in nude mice
- PMID: 20657647
- PMCID: PMC2904700
- DOI: 10.1371/journal.pone.0011583
MMP-9, uPAR and cathepsin B silencing downregulate integrins in human glioma xenograft cells in vitro and in vivo in nude mice
Retraction in
-
Retraction: MMP-9, uPAR and Cathepsin B Silencing Downregulate Integrins in Human Glioma Xenograft Cells In Vitro and In Vivo in Nude Mice.PLoS One. 2025 Jul 11;20(7):e0328092. doi: 10.1371/journal.pone.0328092. eCollection 2025. PLoS One. 2025. PMID: 40644359 Free PMC article. No abstract available.
Abstract
Background: Involvement of MMP-9, uPAR and cathepsin B in adhesion, migration, invasion, proliferation, metastasis and tumor growth has been well established. In the present study, MMP-9, uPAR and cathepsin B genes were downregulated in glioma xenograft cells using shRNA plasmid constructs and we evaluated the involvement of integrins and changes in their adhesion, migration and invasive potential.
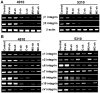
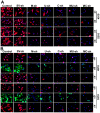
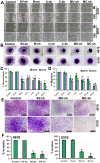
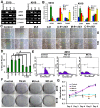
Methodology/principal findings: MMP-9, uPAR and cathepsin B single shRNA plasmid constructs were used to downregulate these molecules in xenograft cells. We also used MMP-9/uPAR and MMP-9/cathepsin B bicistronic constructs to evaluate the cumulative effects. MMP-9, uPAR and cathepsin B downregulation significantly inhibits xenograft cell adhesion to several extracellular matrix proteins. Treatment with MMP-9, uPAR and cathepsin B shRNA of xenografts led to the downregulation of several alpha and beta integrins. In all the assays, we noticed more prominent effects with the bicistronic plasmid constructs when compared to the single plasmid shRNA constructs. FACS analysis demonstrated the expression of alphaVbeta3, alpha6beta1 and alpha9beta1 integrins in xenograft cells. Treatment with bicistronic constructs reduced alphaVbeta3, alpha6beta1 and alpha9beta1 integrin expressions in xenograft injected nude mice. Migration and invasion were also inhibited by MMP-9, uPAR and cathepsin B shRNA treatments as assessed by spheroid migration, wound healing, and Matrigel invasion assays. As expected, bicistronic constructs further inhibited the adhesion, migration and invasive potential of the xenograft cells as compared to individual treatments.
Conclusions/significance: Downregulation of MMP-9, uPAR and cathespin B alone and in combination inhibits adhesion, migration and invasive potential of glioma xenografts by downregulating integrins and associated signaling molecules. Considering the existence of integrin inhibitor-resistant cancer cells, our study provides a novel and effective approach to inhibiting integrins by downregulating MMP-9, uPAR and cathepsin B in the treatment of glioma.
Conflict of interest statement
Figures




References
-
- Albelda SM, Buck CA. Integrins and other cell adhesion molecules. FASEB J. 1990;4:2868–2880. - PubMed
-
- Hemler ME. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. - PubMed
-
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. - PubMed
-
- Ruoslahti E. Fibronectin and its integrin receptors in cancer. Adv Cancer Res. 1999;76:1–20. - PubMed
-
- Schaller MD, Parsons JT. Focal adhesion kinase and associated proteins. Curr Opin Cell Biol. 1994;6:705–710. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

