Tailored Composite Polymer-Metal Nanoparticles by Miniemulsion Polymerization and Thiol-ene Functionalization
- PMID: 20657708
- PMCID: PMC2908426
- DOI: 10.1002/pola.23917
Tailored Composite Polymer-Metal Nanoparticles by Miniemulsion Polymerization and Thiol-ene Functionalization
Abstract
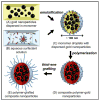
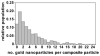
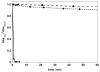
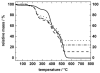
A simple and modular synthetic approach, based on miniemulsion polymerization, has been developed for the fabrication of composite polymer-metal nanoparticle materials. The procedure produces well-defined composite structures consisting of gold, silver or MnFe(2)O(4) nanoparticles (∼10 nm in diameter) encapsulated within larger spherical nanoparticles of poly(divinylbenzene) (∼100 nm in diameter). This methodology readily permits the incorporation of multiple metal domains into a single polymeric particle, while still preserving the useful optical and magnetic properties of the metal nanoparticles. The morphology of the composite particles is retained upon increasing the inorganic content, and also upon redispersion in organic solvents. Finally, the ability to tailor the surface chemistry of the composite nanoparticles and incorporate steric stabilizing groups using simple thiol-ene chemistry is demonstrated.
Figures








Similar articles
-
Polymeric nanoparticles for selective protein recognition by using thiol-ene miniemulsion photopolymerization.J Biomater Sci Polym Ed. 2020 Nov;31(16):2044-2059. doi: 10.1080/09205063.2020.1793705. Epub 2020 Jul 22. J Biomater Sci Polym Ed. 2020. PMID: 32643560
-
Thiol-ene miniemulsion polymerization of a biobased monomer for biomedical applications.Colloids Surf B Biointerfaces. 2017 Nov 1;159:509-517. doi: 10.1016/j.colsurfb.2017.07.043. Epub 2017 Jul 18. Colloids Surf B Biointerfaces. 2017. PMID: 28843199
-
Evaluation of the in vivo acute toxicity of poly(thioether-ester) and superparamagnetic poly(thioether-ester) nanoparticles obtained by thiol-ene miniemulsion polymerization.J Biomed Mater Res B Appl Biomater. 2022 Mar;110(3):702-711. doi: 10.1002/jbm.b.34949. Epub 2021 Oct 7. J Biomed Mater Res B Appl Biomater. 2022. PMID: 34619018
-
A Survey on Synthesis Processes of Structured Materials for Biomedical Applications: Iron-based Magnetic Nanoparticles, Polymeric Materials and Polymerization Processes.Curr Pharm Des. 2015;21(37):5336-58. doi: 10.2174/1381612821666150917093031. Curr Pharm Des. 2015. PMID: 26377657 Review.
-
Morphology Control of Polymer-Inorganic Hybrid Nanomaterials Prepared in Miniemulsion: From Solid Particles to Capsules.Polymers (Basel). 2024 Oct 25;16(21):2997. doi: 10.3390/polym16212997. Polymers (Basel). 2024. PMID: 39518208 Free PMC article. Review.
Cited by
-
Functional, composite polythioether nanoparticles via thiol-alkyne photopolymerization in miniemulsion.Chem Commun (Camb). 2015 Jul 11;51(54):10910-3. doi: 10.1039/c5cc03319e. Chem Commun (Camb). 2015. PMID: 26060848 Free PMC article.
-
Thiol-Mediated Chemoselective Strategies for In Situ Formation of Hydrogels.Gels. 2018 Sep 2;4(3):72. doi: 10.3390/gels4030072. Gels. 2018. PMID: 30674848 Free PMC article. Review.
-
Thiol-Ene Coupling of High Oleic Sunflower Oil towards Application in the Modification of Flexible Polyurethane Foams.Materials (Basel). 2022 Jan 14;15(2):628. doi: 10.3390/ma15020628. Materials (Basel). 2022. PMID: 35057346 Free PMC article.
-
Mesostructured Block Copolymer Nanoparticles: Versatile Templates for Hybrid Inorganic/Organic Nanostructures.Chem Mater. 2012 Nov 13;24(21):4036-4042. doi: 10.1021/cm3011524. Epub 2012 Oct 15. Chem Mater. 2012. PMID: 23335837 Free PMC article.
-
Thiyl Radicals: Versatile Reactive Intermediates for Cyclization of Unsaturated Substrates.Molecules. 2020 Jul 7;25(13):3094. doi: 10.3390/molecules25133094. Molecules. 2020. PMID: 32646036 Free PMC article. Review.
References
-
- Wang D, Salgueiriño-Maceira V, Liz-Marzán LM, Caruso F. Adv Mater. 2002;14:908–912.
- Ouyang J, Chu CW, Szmanda CR, Ma L, Yang Y. Nature Mater. 2004;3:918–922. - PubMed
- Correa-Duarte MA, Salgueirino-Maceira V, Rinaldi A, Giersig M, Liz-Marzan LM. Gold Bull. 2007;40:6–14.
- Beissenhirtz MK, Elnathan R, Weizmann Y, Willner I. Small. 2007;3:375–379. - PubMed
-
- Cao YC, Jin R, Mirkin CA. Science. 2002;297:1536–1540. - PubMed
- Rosi NL, Mirkin CA. Chem Rev. 2005;105:1547–1562. - PubMed
- Rosi NL, Giljohann DA, Thaxton CS, Lytton-Jean AKR, Han MS, Mirkin CA. Science. 2006;312:1027–1030. - PubMed
- Lee JS, Han MS, Mirkin CA. Angew Chem Int Ed. 2007;46:4093–4096. - PubMed
- Bhattacharya R, Patra CR, Verma R, Kumar S, Greipp PR, Mukherjee P. Adv Mater. 2007;19:711–716.
-
- Shan J, Tenhu H. Chem Commun. 2007:4580–4598. - PubMed
-
- Leff DV, Brandt L, Heath JR. Langmuir. 1996;12:4723–4730.
- Newman JDS, Blanchard GJ. Langmuir. 2006;22:5882–5887. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
