Erythropoietin Receptor Signaling Through STAT3 Is Required For Glioma Stem Cell Maintenance
- PMID: 20657792
- PMCID: PMC2906804
- DOI: 10.1177/1947601909356352
Erythropoietin Receptor Signaling Through STAT3 Is Required For Glioma Stem Cell Maintenance
Abstract
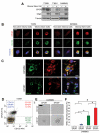
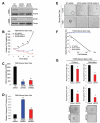
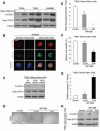
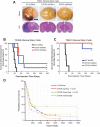
Recombinant erythropoietin (EPO) is a growth factor used in the treatment of chemotherapy-induced anemia, but recent studies suggest that EPO may accelerate cancer growth. Although several cancers express EPO receptors (EPORs), the mechanism by which EPOR promotes tumor growth remains poorly understood. Glioblastomas display a cellular hierarchy of self-renewal and tumor propagation restricted to glioma stem cells (GSCs). We find that GSCs express higher levels of EPOR than matched non-stem glioma cells. Prospective enrichment for EPOR on GSCs increased neurosphere formation, suggesting that EPOR can select for a subset of GSCs with increased self-renewal capacity. Targeting EPOR expression with lentiviral mediated short hairpin RNA (shRNA) reduced GSC growth, survival, and neurosphere formation capacity, defining a crucial role for EPOR in GSC maintenance. We further find that STAT3 is an important mediator of EPOR signals in GSCs. EPOR knockdown attenuated the basal activation of STAT3 present in GSCs, and a small molecule inhibitor of STAT3 reduced GSC growth and survival. EPOR signaling was critical for survival in vivo, as targeting EPOR expression decreased GSC tumorigenic potential. Elevated EPOR expression also associated with poor patient outcome. Thus, EPOR on GSCs promotes tumor growth and may explain the poor survival of cancer patients treated with EPO.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
Figures
References
-
- Central Brain Tumor Registry of the United States (CBTRUS) Statistical report: primary brain tumors in the United States, 1998-2002. Hinsdale, IL: CBTRUS; 2005
-
- Stupp R, Hegi ME, van den Bent MJ, Mason WP, Weller M, Mirimanoff RO, et al. Changing paradigms: an update on the multidisciplinary management of malignant glioma. Oncologist 2006;11:165-80 - PubMed
-
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res 2003;63:5821-8 - PubMed
-
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature 2004;432:396-401 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
