Extreme CD8 T cell requirements for anti-malarial liver-stage immunity following immunization with radiation attenuated sporozoites
- PMID: 20657824
- PMCID: PMC2904779
- DOI: 10.1371/journal.ppat.1000998
Extreme CD8 T cell requirements for anti-malarial liver-stage immunity following immunization with radiation attenuated sporozoites
Abstract
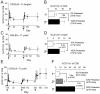
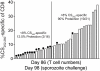
Radiation-attenuated Plasmodium sporozoites (RAS) are the only vaccine shown to induce sterilizing protection against malaria in both humans and rodents. Importantly, these "whole-parasite" vaccines are currently under evaluation in human clinical trials. Studies with inbred mice reveal that RAS-induced CD8 T cells targeting liver-stage parasites are critical for protection. However, the paucity of defined T cell epitopes for these parasites has precluded precise understanding of the specific characteristics of RAS-induced protective CD8 T cell responses. Thus, it is not known whether quantitative or qualitative differences in RAS-induced CD8 T cell responses underlie the relative resistance or susceptibility of immune inbred mice to sporozoite challenge. Moreover, whether extraordinarily large CD8 T cell responses are generated and required for protection following RAS immunization, as has been described for CD8 T cell responses following single-antigen subunit vaccination, remains unknown. Here, we used surrogate T cell activation markers to identify and track whole-parasite, RAS-vaccine-induced effector and memory CD8 T cell responses. Our data show that the differential susceptibility of RAS-immune inbred mouse strains to Plasmodium berghei or P. yoelii sporozoite challenge does not result from host- or parasite-specific decreases in the CD8 T cell response. Moreover, the surrogate activation marker approach allowed us for the first time to evaluate CD8 T cell responses and protective immunity following RAS-immunization in outbred hosts. Importantly, we show that compared to a protective subunit vaccine that elicits a CD8 T cell response to a single epitope, diversifying the targeted antigens through whole-parasite RAS immunization only minimally, if at all, reduced the numerical requirements for memory CD8 T cell-mediated protection. Thus, our studies reveal that extremely high frequencies of RAS-induced memory CD8 T cells are required, but may not suffice, for sterilizing anti-Plasmodial immunity. These data provide new insights into protective CD8 T cell responses elicited by RAS-immunization in genetically diverse hosts, information with relevance to developing attenuated whole-parasite vaccines.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Long lived liver-resident memory T cells of biased specificities for abundant sporozoite antigens drive malaria protection by radiation-attenuated sporozoite vaccination.PLoS Pathog. 2025 May 27;21(5):e1012731. doi: 10.1371/journal.ppat.1012731. eCollection 2025 May. PLoS Pathog. 2025. PMID: 40424562 Free PMC article.
-
Long term protection after immunization with P. berghei sporozoites correlates with sustained IFNγ responses of hepatic CD8+ memory T cells.PLoS One. 2012;7(5):e36508. doi: 10.1371/journal.pone.0036508. Epub 2012 May 1. PLoS One. 2012. PMID: 22563506 Free PMC article.
-
CD8 T cell independent immunity after single dose infection-treatment-vaccination (ITV) against Plasmodium yoelii.Vaccine. 2014 Jan 16;32(4):483-91. doi: 10.1016/j.vaccine.2013.11.058. Epub 2013 Dec 7. Vaccine. 2014. PMID: 24321740 Free PMC article.
-
Route of administration of attenuated sporozoites is instrumental in rendering immunity against Plasmodia infection.Vaccine. 2016 Jun 14;34(28):3229-34. doi: 10.1016/j.vaccine.2016.04.095. Epub 2016 May 6. Vaccine. 2016. PMID: 27160038 Review.
-
Current Challenges in the Identification of Pre-Erythrocytic Malaria Vaccine Candidate Antigens.Front Immunol. 2020 Feb 21;11:190. doi: 10.3389/fimmu.2020.00190. eCollection 2020. Front Immunol. 2020. PMID: 32153565 Free PMC article. Review.
Cited by
-
In vivo imaging of CD8+ T cell-mediated elimination of malaria liver stages.Proc Natl Acad Sci U S A. 2013 May 28;110(22):9090-5. doi: 10.1073/pnas.1303858110. Epub 2013 May 14. Proc Natl Acad Sci U S A. 2013. PMID: 23674673 Free PMC article.
-
Mechanisms of stage-transcending protection following immunization of mice with late liver stage-arresting genetically attenuated malaria parasites.PLoS Pathog. 2015 May 14;11(5):e1004855. doi: 10.1371/journal.ppat.1004855. eCollection 2015 May. PLoS Pathog. 2015. PMID: 25974076 Free PMC article.
-
Lung endothelial cell antigen cross-presentation to CD8+T cells drives malaria-associated lung injury.Nat Commun. 2019 Sep 18;10(1):4241. doi: 10.1038/s41467-019-12017-8. Nat Commun. 2019. PMID: 31534124 Free PMC article.
-
Vaccine platforms combining circumsporozoite protein and potent immune modulators, rEA or EAT-2, paradoxically result in opposing immune responses.PLoS One. 2011;6(8):e24147. doi: 10.1371/journal.pone.0024147. Epub 2011 Aug 30. PLoS One. 2011. PMID: 21912619 Free PMC article.
-
Acute Plasmodium Infection Promotes Interferon-Gamma-Dependent Resistance to Ebola Virus Infection.Cell Rep. 2020 Mar 24;30(12):4041-4051.e4. doi: 10.1016/j.celrep.2020.02.104. Cell Rep. 2020. PMID: 32209467 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

