Thy-1 attenuates TNF-alpha-activated gene expression in mouse embryonic fibroblasts via Src family kinase
- PMID: 20657842
- PMCID: PMC2906514
- DOI: 10.1371/journal.pone.0011662
Thy-1 attenuates TNF-alpha-activated gene expression in mouse embryonic fibroblasts via Src family kinase
Abstract
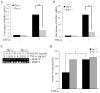
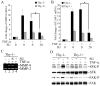
Heterogeneous surface expression of Thy-1 in fibroblasts modulates inflammation and may thereby modulate injury and repair. As a paradigm, patients with idiopathic pulmonary fibrosis, a disease with pathologic features of chronic inflammation, demonstrate an absence of Thy-1 immunoreactivity within areas of fibrotic activity (fibroblast foci) in contrast to the predominant Thy-1 expressing fibroblasts in the normal lung. Likewise, Thy-1 deficient mice display more severe lung fibrosis in response to an inflammatory injury than wildtype littermates. We investigated the role of Thy-1 in the response of fibroblasts to the pro-inflammatory cytokine TNF-alpha. Our study demonstrates distinct profiles of TNF-alpha-activated gene expression in Thy-1 positive (Thy-1+) and negative (Thy-1-) subsets of mouse embryonic fibroblasts (MEF). TNF-alpha induced a robust activation of MMP-9, ICAM-1, and the IL-8 promoter driven reporter in Thy-1- MEFs, in contrast to only a modest increase in Thy-1+ counterparts. Consistently, ectopic expression of Thy-1 in Thy-1- MEFs significantly attenuated TNF-alpha-activated gene expression. Mechanistically, TNF-alpha activated Src family kinase (SFK) only in Thy-1- MEFs. Blockade of SFK activation abrogated TNF-alpha-activated gene expression in Thy-1- MEFs, whereas restoration of SFK activation rescued the TNF-alpha response in Thy-1+ MEFs. Our findings suggest that Thy-1 down-regulates TNF-alpha-activated gene expression via interfering with SFK- and NF-kappaB-mediated transactivation. The current study provides a novel mechanistic insight to the distinct roles of fibroblast Thy-1 subsets in inflammation.
Conflict of interest statement
Figures

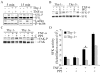

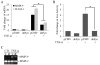

Similar articles
-
Inhibition of ICAM-1 gene expression, monocyte adhesion and cancer cell invasion by targeting IKK complex: molecular and functional study of novel alpha-methylene-gamma-butyrolactone derivatives.Carcinogenesis. 2004 Oct;25(10):1925-34. doi: 10.1093/carcin/bgh211. Epub 2004 Jun 24. Carcinogenesis. 2004. PMID: 15217903
-
Hypoxia-induced DNA hypermethylation in human pulmonary fibroblasts is associated with Thy-1 promoter methylation and the development of a pro-fibrotic phenotype.Respir Res. 2012 Aug 31;13(1):74. doi: 10.1186/1465-9921-13-74. Respir Res. 2012. PMID: 22938014 Free PMC article.
-
Differential expression of interleukin 1 alpha by Thy-1+ and Thy-1- lung fibroblast subpopulations: enhancement of interleukin 1 alpha production by tumor necrosis factor-alpha.Eur J Immunol. 1990 Aug;20(8):1723-7. doi: 10.1002/eji.1830200815. Eur J Immunol. 1990. PMID: 1976521
-
c-Src-dependent transactivation of PDGFR contributes to TNF-α-induced MMP-9 expression and functional impairment in osteoblasts.Bone. 2014 Mar;60:186-97. doi: 10.1016/j.bone.2013.12.014. Epub 2013 Dec 18. Bone. 2014. PMID: 24361597
-
Roles and regulation of Thy-1, a context-dependent modulator of cell phenotype.Biofactors. 2009 May-Jun;35(3):258-65. doi: 10.1002/biof.41. Biofactors. 2009. PMID: 19422052 Free PMC article. Review.
Cited by
-
FAK Inhibition Attenuates Corneal Fibroblast Differentiation In Vitro.Biomolecules. 2021 Nov 12;11(11):1682. doi: 10.3390/biom11111682. Biomolecules. 2021. PMID: 34827680 Free PMC article.
-
Elevated expression of long intergenic non-coding RNA HOTAIR in a basal-like variant of MCF-7 breast cancer cells.Mol Carcinog. 2015 Dec;54(12):1656-67. doi: 10.1002/mc.22237. Epub 2014 Oct 18. Mol Carcinog. 2015. PMID: 25328122 Free PMC article.
-
Induction of a novel isoform of the lncRNA HOTAIR in Claudin-low breast cancer cells attached to extracellular matrix.Mol Oncol. 2017 Dec;11(12):1698-1710. doi: 10.1002/1878-0261.12133. Epub 2017 Oct 30. Mol Oncol. 2017. PMID: 28846832 Free PMC article.
-
Src-mediated morphology transition of lung cancer cells in three-dimensional organotypic culture.Cancer Cell Int. 2013 Feb 14;13(1):16. doi: 10.1186/1475-2867-13-16. Cancer Cell Int. 2013. PMID: 23409704 Free PMC article.
-
Induction of long intergenic non-coding RNA HOTAIR in lung cancer cells by type I collagen.J Hematol Oncol. 2013 May 13;6:35. doi: 10.1186/1756-8722-6-35. J Hematol Oncol. 2013. PMID: 23668363 Free PMC article.
References
-
- Rege TA, Hagood JS. Thy-1 as a regulator of cell-cell and cell-matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. FASEB J. 2006;20:1045–1054. - PubMed
-
- Rege TA, Pallero MA, Gomez C, Grenett HE, Murphy-Ullrich JE, et al. Thy-1, via its GPI anchor, modulates Src family kinase and focal adhesion kinase phosphorylation and subcellular localization, and fibroblast migration, in response to thrombospondin-1/hep I. Exp Cell Res. 2006;312:3752–3767. - PubMed
-
- Barker TH, Pallero MA, MacEwen MW, Tilden SG, Woods A, et al. Thrombospondin-1-induced focal adhesion disassembly in fibroblasts requires Thy-1 surface expression, lipid raft integrity, and Src activation. J Biol Chem. 2004;279:23510–23516. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

