High-throughput identification of chemical inhibitors of E. coli Group 2 capsule biogenesis as anti-virulence agents
- PMID: 20657847
- PMCID: PMC2906519
- DOI: 10.1371/journal.pone.0011642
High-throughput identification of chemical inhibitors of E. coli Group 2 capsule biogenesis as anti-virulence agents
Abstract
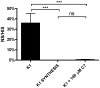
Rising antibiotic resistance among Escherichia coli, the leading cause of urinary tract infections (UTIs), has placed a new focus on molecular pathogenesis studies, aiming to identify new therapeutic targets. Anti-virulence agents are attractive as chemotherapeutics to attenuate an organism during disease but not necessarily during benign commensalism, thus decreasing the stress on beneficial microbial communities and lessening the emergence of resistance. We and others have demonstrated that the K antigen capsule of E. coli is a preeminent virulence determinant during UTI and more invasive diseases. Components of assembly and export are highly conserved among the major K antigen capsular types associated with UTI-causing E. coli and are distinct from the capsule biogenesis machinery of many commensal E. coli, making these attractive therapeutic targets. We conducted a screen for anti-capsular small molecules and identified an agent designated "C7" that blocks the production of K1 and K5 capsules, unrelated polysaccharide types among the Group 2-3 capsules. Herein lies proof-of-concept that this screen may be implemented with larger chemical libraries to identify second-generation small-molecule inhibitors of capsule biogenesis. These inhibitors will lead to a better understanding of capsule biogenesis and may represent a new class of therapeutics.
Conflict of interest statement
Figures
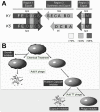
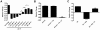
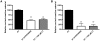
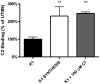
References
-
- Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Dis Mon. 2003;49:53–70. - PubMed
-
- Litwin M, Saigal C. Introduction. In: Litwin M, Saigal C, editors. Urologic Diseases in America. 07–5512. Washington, DC: NIH publication; 2007. pp. 3–7.
-
- Gupta K, Hooton TM, Stamm WE. Isolation of fluoroquinolone-resistant rectal Escherichia coli after treatment of acute uncomplicated cystitis. J Antimicrob Chemother. 2005;56:243–246. - PubMed
-
- Gupta K, Scholes D, Stamm WE. Increasing prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in women. JAMA. 1999;281:736–738. - PubMed
-
- Kahlmeter G. The ECO.SENS Project: a prospective, multinational, multicentre epidemiological survey of the prevalence and antimicrobial susceptibility of urinary tract pathogens—interim report. J Antimicrob Chemother : . 2000;46(Suppl 1):15-22; discussion 63–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

