Parenteral delivery of HPβCD: effects on drug-HSA binding
- PMID: 20658211
- PMCID: PMC2974121
- DOI: 10.1208/s12249-010-9482-0
Parenteral delivery of HPβCD: effects on drug-HSA binding
Abstract
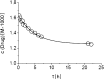
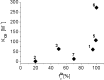
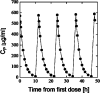
It is thought that cyclodextrins, such as 2-hydroxypropyl-β-cyclodextrin (HPβCD), will at high concentration affect pharmacokinetics of drugs through competitive binding with plasma proteins. Albumin is the major component of plasma proteins responsible for plasma protein binding. The purpose of this study was to evaluate in vitro the competitive binding of drugs between human serum albumin (HSA) and HPβCD in isotonic pH 7.4 phosphate buffer saline solution (PBS) at ambient temperature. Eight model drugs were selected based on their physicochemical properties and ability to form complexes with HSA and HPβCD. The drug/HPβCD stability constants (K(1:1)) were determined by the phase-solubility method and HSA/HPβCD competitive binding determined by an equilibrium dialysis method. Protein binding of drugs that are both strongly protein bound and have high affinity to HPβCD (i.e., have high K(1:1) value) is most likely to be affected by parenterally administered HPβCD. However, this in vitro study indicates that even for those drugs single parenteral dose of HPβCD has to be as high as 70 g to have detectable effect on their protein binding. Weakly protein bound drugs and drugs with low affinity towards HPβCD are insensitive to the cyclodextrin presence regardless their lipophilic properties.
Figures
Similar articles
-
The effect of parenterally administered cyclodextrins on the pharmacokinetics of coadministered drugs.J Pharm Sci. 2012 Dec;101(12):4402-8. doi: 10.1002/jps.23329. Epub 2012 Oct 2. J Pharm Sci. 2012. PMID: 23033143
-
Dihydroartemisinin-cyclodextrin complexation: solubility and stability.Arch Pharm Res. 2009 Jan;32(1):155-65. doi: 10.1007/s12272-009-1130-4. Epub 2009 Jan 29. Arch Pharm Res. 2009. PMID: 19183889
-
Physicochemical characterization of berberine chloride: a perspective in the development of a solution dosage form for oral delivery.AAPS PharmSciTech. 2010 Sep;11(3):1466-75. doi: 10.1208/s12249-010-9520-y. Epub 2010 Sep 15. AAPS PharmSciTech. 2010. PMID: 20842541 Free PMC article.
-
Effect of beta-cyclodextrin and hydroxypropyl beta-cyclodextrin complexation on physicochemical properties and antimicrobial activity of cefdinir.J Pharm Biomed Anal. 2008 Jul 15;47(3):535-40. doi: 10.1016/j.jpba.2008.02.006. Epub 2008 Feb 15. J Pharm Biomed Anal. 2008. PMID: 18367363 Review.
-
Efficacy and Safety Profile of Diclofenac/Cyclodextrin and Progesterone/Cyclodextrin Formulations: A Review of the Literature Data.Drugs R D. 2016 Jun;16(2):129-40. doi: 10.1007/s40268-016-0123-2. Drugs R D. 2016. PMID: 26939533 Free PMC article. Review.
References
-
- Jansook P, Kurkov SV, Loftsson T. Cyclodextrins as solubilizers: formation of complex aggregates. J Pharm Sci. 2010;99(2):719–729. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

