Deficiency in core circadian protein Bmal1 is associated with a prothrombotic and vascular phenotype
- PMID: 20658528
- PMCID: PMC2982145
- DOI: 10.1002/jcp.22314
Deficiency in core circadian protein Bmal1 is associated with a prothrombotic and vascular phenotype
Abstract
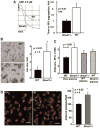
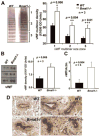
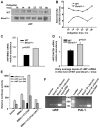
Aging is associated with both the disturbances of circadian rhythms and a prothrombotic phenotype. It remains poorly understood how the circadian system regulates thrombosis, a critical outcome of aging-related cardiovascular disease. Using multiple in vivo models, we now show that mice with genetic ablation of the core clock gene Bmal1, which display pre-mature aging, have a dramatic prothrombotic phenotype. This phenotype is mechanistically linked to changes in the regulation of key risk factors for cardiovascular disease. These include circulating vWF, fibrinogen, and PAI-1, all of which are significantly elevated in Bmal1(-/-) mice. We also show that major circadian transcriptional regulators CLOCK and Bmal1 directly regulate the activity of vWF promoter and that lack of Bmal1 results in upregulation of vWF both at mRNA and protein level. Here we report a direct regulation of vWF expression in endothelial cells by biological clock gene Bmal1. This study establishes a mechanistic connection between Bmal1 and cardiovascular phenotype.
Figures


References
-
- Bunger MK, Walisser JA, Sullivan R, Manley PA, Moran SM, Kalscheur VL, Colman RJ, Bradfield CA. Progressive arthropathy in mice with a targeted disruption of the Mop3/Bmal-1 locus. Genesis. 2005;41:122–132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

