Down-regulation of Notch-1 is associated with Akt and FoxM1 in inducing cell growth inhibition and apoptosis in prostate cancer cells
- PMID: 20658545
- PMCID: PMC3792569
- DOI: 10.1002/jcb.22770
Down-regulation of Notch-1 is associated with Akt and FoxM1 in inducing cell growth inhibition and apoptosis in prostate cancer cells
Retraction in
-
Retraction: "Down-regulation of Notch-1 is associated with Akt and FoxM1 in inducing cell growth inhibition and apoptosis in prostate cancer cells" by Wang et al.J Cell Biochem. 2016 Aug;117(8):1962. doi: 10.1002/jcb.25587. J Cell Biochem. 2016. PMID: 27301889 Free PMC article.
Abstract
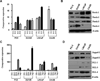
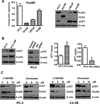
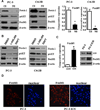
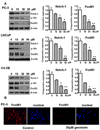
Although many studies have been done to uncover the mechanisms by which down-regulation of Notch-1 exerts its anti-tumor activity against a variety of human malignancies, the precise molecular mechanisms remain unclear. In the present study, we investigated the cellular consequence of Notch-1 down-regulation and also assessed the molecular consequence of Notch-1-mediated alterations of its downstream targets on cell viability and apoptosis in prostate cancer (PCa) cells. We found that the down-regulation of Notch-1 led to the inhibition of cell growth and induction of apoptosis, which was mechanistically linked with down-regulation of Akt and FoxM1, suggesting for the first time that Akt and FoxM1 are downstream targets of Notch-1 signaling. Moreover, we found that a "natural agent" (genistein) originally discovered from soybean could cause significant reduction in cell viability and induced apoptosis of PCa cells, which was consistent with down-regulation of Notch-1, Akt, and FoxM1. These results suggest that down-regulation of Notch-1 by novel agents could become a newer approach for the prevention of tumor progression and/or treatment, which is likely to be mediated via inactivation of Akt and FoxM1 signaling pathways in PCa.
Figures

Similar articles
-
Down-regulation of Notch-1 and Jagged-1 inhibits prostate cancer cell growth, migration and invasion, and induces apoptosis via inactivation of Akt, mTOR, and NF-kappaB signaling pathways.J Cell Biochem. 2010 Mar 1;109(4):726-36. doi: 10.1002/jcb.22451. J Cell Biochem. 2010. Retraction in: J Cell Biochem. 2016 Aug;117(8):1960. doi: 10.1002/jcb.25585. PMID: 20052673 Retracted.
-
Akt-mTOR signaling is involved in Notch-1-mediated glioma cell survival and proliferation.Oncol Rep. 2010 May;23(5):1443-7. doi: 10.3892/or_00000782. Oncol Rep. 2010. PMID: 20372862
-
3,3'-Diindolylmethane potentiates paclitaxel-induced antitumor effects on gastric cancer cells through the Akt/FOXM1 signaling cascade.Oncol Rep. 2015 Apr;33(4):2031-6. doi: 10.3892/or.2015.3758. Epub 2015 Jan 28. Oncol Rep. 2015. PMID: 25633416
-
Role of CSL-dependent and independent Notch signaling pathways in cell apoptosis.Apoptosis. 2016 Jan;21(1):1-12. doi: 10.1007/s10495-015-1188-z. Apoptosis. 2016. PMID: 26496776 Review.
-
Forkhead box M1 transcription factor: a novel target for cancer therapy.Cancer Treat Rev. 2010 Apr;36(2):151-6. doi: 10.1016/j.ctrv.2009.11.006. Epub 2009 Dec 22. Cancer Treat Rev. 2010. PMID: 20022709 Free PMC article. Review.
Cited by
-
Over-expression of FoxM1 leads to epithelial-mesenchymal transition and cancer stem cell phenotype in pancreatic cancer cells.J Cell Biochem. 2011 Sep;112(9):2296-306. doi: 10.1002/jcb.23150. J Cell Biochem. 2011. Retraction in: J Cell Biochem. 2016 Aug;117(8):1963. doi: 10.1002/jcb.25588. PMID: 21503965 Free PMC article. Retracted.
-
High FOXM1 expression was associated with bladder carcinogenesis.Tumour Biol. 2013 Apr;34(2):1131-8. doi: 10.1007/s13277-013-0654-x. Epub 2013 Jan 17. Tumour Biol. 2013. PMID: 23325617
-
Notch promotes tumor metastasis in a prostate-specific Pten-null mouse model.J Clin Invest. 2016 Jul 1;126(7):2626-41. doi: 10.1172/JCI84637. Epub 2016 Jun 13. J Clin Invest. 2016. PMID: 27294523 Free PMC article.
-
Retraction: "Down-regulation of Notch-1 is associated with Akt and FoxM1 in inducing cell growth inhibition and apoptosis in prostate cancer cells" by Wang et al.J Cell Biochem. 2016 Aug;117(8):1962. doi: 10.1002/jcb.25587. J Cell Biochem. 2016. PMID: 27301889 Free PMC article.
-
Hypoxia, notch signalling, and prostate cancer.Nat Rev Urol. 2013 Jul;10(7):405-13. doi: 10.1038/nrurol.2013.110. Epub 2013 May 28. Nat Rev Urol. 2013. PMID: 23712204 Free PMC article. Review.
References
-
- Banerjee S, Zhang Y, Ali S, Bhuiyan M, Wang Z, Chiao PJ, Philip PA, Abbruzzese J, Sarkar FH. Molecular evidence for increased antitumor activity of gemcitabine by genistein in vitro and in vivo using an orthotopic model of pancreatic cancer. Cancer Res. 2005;65:9064–9072. - PubMed
-
- Banerjee S, Hussain M, Wang Z, Saliganan A, Che M, Bonfil D, Cher M, Sarkar FH. In vitro and in vivo molecular evidence for better therapeutic efficacy of ABT-627 and taxotere combination in prostate cancer. Cancer Res. 2007a;67:3818–3826. - PubMed
-
- Banerjee S, Zhang Y, Wang Z, Che M, Chiao PJ, Abbruzzese JL, Sarkar FH. In vitro and in vivo molecular evidence of genistein action in augmenting the efficacy of cisplatin in pancreatic cancer. Int J Cancer. 2007b;120:906–917. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

