Alzheimer's disease neurofibrillary degeneration: pivotal and multifactorial
- PMID: 20658985
- PMCID: PMC3092440
- DOI: 10.1042/BST0380962
Alzheimer's disease neurofibrillary degeneration: pivotal and multifactorial
Abstract
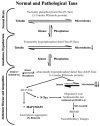
Independent of the aetiology, AD (Alzheimer's disease) neurofibrillary degeneration of abnormally hyperphosphorylated tau, a hallmark of AD and related tauopathies, is apparently required for the clinical expression of the disease and hence is a major therapeutic target for drug development. However, AD is multifactorial and heterogeneous and probably involves several different aetiopathogenic mechanisms. On the basis of CSF (cerebrospinal fluid) levels of Abeta(1-42) (where Abeta is amyloid beta-peptide), tau and ubiquitin, five different subgroups, each with its own clinical profile, have been identified. A successful development of rational therapeutic disease-modifying drugs for AD will require understanding of the different aetiopathogenic mechanisms involved and stratification of AD patients by different disease subgroups in clinical trials. We have identified a novel aetiopathogenic mechanism of AD which is initiated by the cleavage of SET, also known as inhibitor-2 (I(2)(PP2A)) of PP2A (protein phosphatase 2A) at Asn(175) into N-terminal (I(2NTF)) and C-terminal (I(2CTF)) halves and their translocation from the neuronal nucleus to the cytoplasm. AAV1 (adeno-associated virus 1)-induced expression of I(2CTF) in rat brain induces inhibition of PP2A activity, abnormal hyperphosphorylation of tau, neurodegeneration and cognitive impairment in rats. Restoration of PP2A activity by inhibition of the cleavage of I(2)(PP2A)/SET offers a promising therapeutic opportunity in AD with this aetiopathogenic mechanism.
Figures
References
-
- Glenner GG, Wong CW. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. - PubMed
-
- Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule-associated protein tau: a component of Alzheimer paired helical filaments. J Biol Chem. 1986;261:6084–6089. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

