Alloimmune activation enhances innate tissue inflammation/injury in a mouse model of liver ischemia/reperfusion injury
- PMID: 20659085
- PMCID: PMC3655759
- DOI: 10.1111/j.1600-6143.2010.03205.x
Alloimmune activation enhances innate tissue inflammation/injury in a mouse model of liver ischemia/reperfusion injury
Abstract
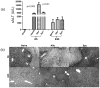
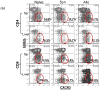
The deleterious sensitization to donor MHC Ags represents one of the most challenging problems in clinical organ transplantation. Although the role of effector/memory T cells in the rejection cascade has been extensively studied, it remains unknown whether and how these 'Ag-specific' cells influence host innate immunity, such as tissue inflammation associated with ischemia and reperfusion injury (IRI). In this study, we analyzed how allogeneic skin transplant (Tx) affected the sequel of host's own liver damage induced by partial warm ischemia and reperfusion. Our data clearly showed that allo-Tx recipients had increased inflammatory response against IR insult in their native livers, as evidenced by significantly more severe hepatocelluar damage, compared with syngeneic Tx recipient controls, and determined by serum ALT levels, liver histology (Suzuki's score) and intrahepatic proinflammatory gene inductions (TNF-alpha, IL-1beta and CXCL10). The CD4 T cells, but neither CD8 nor NK cells, mediated the detrimental effect of allo-Ag sensitization in liver IRI. Furthermore, CD154, but not IFN-gamma, was the key mechanism in allo-Tx recipients to facilitate IR-triggered liver damage. These results provide new evidence that alloreactive CD4 T cells are capable of enhancing innate tissue inflammation and organ injury via an Ag-nonspecific CD154-dependent but IFN-gamma independent mechanism.
Figures


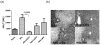



Similar articles
-
CD4 T cells promote tissue inflammation via CD40 signaling without de novo activation in a murine model of liver ischemia/reperfusion injury.Hepatology. 2009 Nov;50(5):1537-46. doi: 10.1002/hep.23153. Hepatology. 2009. PMID: 19670423 Free PMC article.
-
Recipient T cell TIM-3 and hepatocyte galectin-9 signalling protects mouse liver transplants against ischemia-reperfusion injury.J Hepatol. 2015 Mar;62(3):563-72. doi: 10.1016/j.jhep.2014.10.034. Epub 2014 Oct 31. J Hepatol. 2015. PMID: 25450716 Free PMC article.
-
CXCR3+CD4+ T cells mediate innate immune function in the pathophysiology of liver ischemia/reperfusion injury.J Immunol. 2006 May 15;176(10):6313-22. doi: 10.4049/jimmunol.176.10.6313. J Immunol. 2006. PMID: 16670343
-
CD4+ T Cell NRF2 Signaling Improves Liver Transplantation Outcomes by Modulating T Cell Activation and Differentiation.Antioxid Redox Signal. 2023 Mar;38(7-9):670-683. doi: 10.1089/ars.2022.0094. Epub 2023 Mar 1. Antioxid Redox Signal. 2023. PMID: 36070449 Free PMC article. Review.
-
New therapeutic concepts against ischemia-reperfusion injury in organ transplantation.Expert Rev Clin Immunol. 2023 Jul-Dec;19(10):1205-1224. doi: 10.1080/1744666X.2023.2240516. Epub 2023 Jul 28. Expert Rev Clin Immunol. 2023. PMID: 37489289 Free PMC article. Review.
Cited by
-
Liver ischemia and reperfusion injury: new insights into mechanisms of innate-adaptive immune-mediated tissue inflammation.Am J Transplant. 2011 Aug;11(8):1563-9. doi: 10.1111/j.1600-6143.2011.03579.x. Epub 2011 Jun 10. Am J Transplant. 2011. PMID: 21668640 Free PMC article. Review.
-
Effect of the purinergic inhibitor oxidized ATP in a model of islet allograft rejection.Diabetes. 2013 May;62(5):1665-75. doi: 10.2337/db12-0242. Epub 2013 Jan 11. Diabetes. 2013. PMID: 23315496 Free PMC article.
-
Long-term heart transplant survival by targeting the ionotropic purinergic receptor P2X7.Circulation. 2013 Jan 29;127(4):463-75. doi: 10.1161/CIRCULATIONAHA.112.123653. Epub 2012 Dec 18. Circulation. 2013. PMID: 23250993 Free PMC article.
-
Sotrastaurin, a protein kinase C inhibitor, ameliorates ischemia and reperfusion injury in rat orthotopic liver transplantation.Am J Transplant. 2011 Nov;11(11):2499-507. doi: 10.1111/j.1600-6143.2011.03700.x. Epub 2011 Aug 30. Am J Transplant. 2011. PMID: 21883905 Free PMC article.
-
CD154 blockade alters innate immune cell recruitment and programs alloreactive CD8+ T cells into KLRG-1(high) short-lived effector T cells.PLoS One. 2012;7(7):e40559. doi: 10.1371/journal.pone.0040559. Epub 2012 Jul 5. PLoS One. 2012. PMID: 22792369 Free PMC article.
References
-
- Kupiec-Weglinski JW. Graft rejection in sensitized recipients. Ann Transplant. 1996;1(1):34–40. - PubMed
-
- Sablinki T, Hancock WW, Tilney NL, Kupiec-Weglinski JW. Biology of vascularized organ allograft rejection in sensitized recipients. Transplant Rev. 1990;4:108–120.
-
- Baid S, Saidman SL, Tolkoff-Rubin N, Williams WW, Delmonico FL, Cosimi AB, et al. Managing the highly sensitized transplant recipient and B cell tolerance. Curr Opin Immunol. 2001;13(5):577–581. - PubMed
-
- Brook MO, Wood KJ, Jones ND. The impact of memory T cells on rejection and the induction of tolerance. Transplantation. 2006;82(1):1–9. - PubMed
-
- Valujskikh A. The challenge of inhibiting alloreactive T-cell memory. Am J Transplant. 2006;6(4):647–651. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

