Damage of Streptococcus mutans biofilms by carolacton, a secondary metabolite from the myxobacterium Sorangium cellulosum
- PMID: 20659313
- PMCID: PMC2915981
- DOI: 10.1186/1471-2180-10-199
Damage of Streptococcus mutans biofilms by carolacton, a secondary metabolite from the myxobacterium Sorangium cellulosum
Abstract
Background: Streptococcus mutans is a major pathogen in human dental caries. One of its important virulence properties is the ability to form biofilms (dental plaque) on tooth surfaces. Eradication of such biofilms is extremely difficult. We therefore screened a library of secondary metabolites from myxobacteria for their ability to damage biofilms of S. mutans.
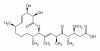
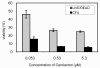
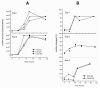
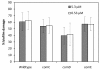
Results: Here we show that carolacton, a secondary metabolite isolated from Sorangium cellulosum, has high antibacterial activity against biofilms of S. mutans. Planktonic growth of bacteria was only slightly impaired and no acute cytotoxicity against mouse fibroblasts could be observed. Carolacton caused death of S. mutans biofilm cells, elongation of cell chains, and changes in cell morphology. At a concentration of 10 nM carolacton, biofilm damage was already at 35% under anaerobic conditions. A knock-out mutant for comD, encoding a histidine kinase specific for the competence stimulating peptide (CSP), was slightly less sensitive to carolacton than the wildtype. Expression of the competence related alternate sigma factor ComX was strongly reduced by carolacton, as determined by a pcomX luciferase reporter strain.
Conclusions: Carolacton possibly interferes with the density dependent signalling systems in S. mutans and may represent a novel approach for the prevention of dental caries.
Figures



References
-
- Costerton JW, Montanaro L, Arciola CR. Bacterial communications in implant infections: a target for an intelligence war. Int J Artif Organs. 2007;30:757–763. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

