Gp91phox (NOX2) in classically activated microglia exacerbates traumatic brain injury
- PMID: 20659322
- PMCID: PMC2917406
- DOI: 10.1186/1742-2094-7-41
Gp91phox (NOX2) in classically activated microglia exacerbates traumatic brain injury
Abstract
Background: We hypothesized that gp91phox (NOX2), a subunit of NADPH oxidase, generates superoxide anion (O2-) and has a major causative role in traumatic brain injury (TBI). To evaluate the functional role of gp91phox and reactive oxygen species (ROS) on TBI, we carried out controlled cortical impact in gp91phox knockout mice (gp91phox-/-). We also used a microglial cell line to determine the activated cell phenotype that contributes to gp91phox generation.
Methods: Unilateral TBI was induced in gp91phox-/- and wild-type (Wt) mice (C57/B6J) (25-30 g). The expression and roles of gp91phox after TBI were investigated using immunoblotting and staining techniques. Levels of O2- and peroxynitrite were determined in situ in the mouse brain. The activated phenotype in microglia that expressed gp91phox was determined in a microglial cell line, BV-2, in the presence of IFNgamma or IL-4.
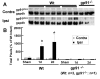
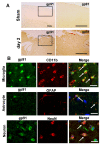
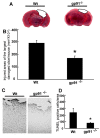
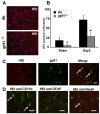
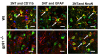
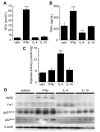
Results: Gp91phox expression increased mainly in amoeboid-shaped microglial cells of the ipsilateral hemisphere of Wt mice after TBI. The contusion area, number of TUNEL-positive cells, and amount of O2- and peroxynitrite metabolites produced were less in gp91phox-/- mice than in Wt. In the presence of IFNgamma, BV-2 cells had increased inducible nitric oxide synthase and nitric oxide levels, consistent with a classical activated phenotype, and drastically increased expression of gp91phox.
Conclusions: Classical activated microglia promote ROS formation through gp91phox and have an important role in brain damage following TBI. Modulating gp91phox and gp91phox -derived ROS may provide a new therapeutic strategy in combating post-traumatic brain injury.
Figures
References
-
- Tyurin VA, Tyurina YY, Borisenko GG, Sokolova TV, Ritov VB, Quinn PJ, Rose M, Kochanek P, Graham SH, Kagan VE. Oxidative stress following traumatic brain injury in rats: quantitation of biomarkers and detection of free radical intermediates. J Neurochem. 2000;75:2178–2189. doi: 10.1046/j.1471-4159.2000.0752178.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

