MYB suppresses differentiation and apoptosis of human breast cancer cells
- PMID: 20659323
- PMCID: PMC2949644
- DOI: 10.1186/bcr2614
MYB suppresses differentiation and apoptosis of human breast cancer cells
Abstract
Introduction: MYB is highly expressed in estrogen receptor positive (ER + ve) breast tumours and tumour cell lines. We recently demonstrated that MYB is essential for the proliferation of ER + ve breast cancer cells, and have now investigated its role in mammary epithelial differentiation.
Methods: MCF-7 breast cancer cells were treated with sodium butyrate, vitamin E succinate or 12-O-tetradecanoylphorbol-13-acetate to induce differentiation as measured by Nile Red staining of lipid droplets and β-casein expression. The non-tumorigenic murine mammary epithelial cell (MEC) line, HC11, was induced to differentiate with lactogenic hormones. MYB levels were manipulated by inducible lentiviral shRNA-mediated knockdown and retroviral overexpression.
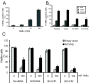
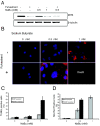
Results: We found that MYB expression decreases following chemically-induced differentiation of the human breast cancer cell line MCF-7, and hormonally-induced differentiation of a non-tumorigenic murine mammary epithelial cell (MEC) line, HC11. We also found that shRNA-mediated MYB knockdown initiated differentiation of breast cancer cells, and greatly sensitised them to the differentiative and pro-apoptotic effects of differentiation-inducing agents (DIAs). Sensitisation to the pro-apoptotic effects DIAs is mediated by decreased expression of BCL2, which we show here is a direct MYB target in breast cancer cells. Conversely, enforced expression of MYB resulted in the cells remaining in an undifferentiated state, with concomitant suppression of apoptosis, in the presence of DIAs.
Conclusions: Taken together, these data imply that MYB function is critical in regulating the balance between proliferation, differentiation, and apoptosis in MECs. Moreover, our findings suggest MYB may be a viable therapeutic target in breast cancer and suggest specific approaches for exploiting this possibility.
Figures






Similar articles
-
CDK9 inhibitors selectively target estrogen receptor-positive breast cancer cells through combined inhibition of MYB and MCL-1 expression.Oncotarget. 2016 Feb 23;7(8):9069-83. doi: 10.18632/oncotarget.6997. Oncotarget. 2016. PMID: 26812885 Free PMC article.
-
Intrinsic mechanism of estradiol-induced apoptosis in breast cancer cells resistant to estrogen deprivation.J Natl Cancer Inst. 2005 Dec 7;97(23):1746-59. doi: 10.1093/jnci/dji400. J Natl Cancer Inst. 2005. PMID: 16333030
-
Identification and regulation of c-Myb target genes in MCF-7 cells.BMC Cancer. 2011 Jan 25;11:30. doi: 10.1186/1471-2407-11-30. BMC Cancer. 2011. PMID: 21261996 Free PMC article.
-
Buthionine sulfoximine sensitizes antihormone-resistant human breast cancer cells to estrogen-induced apoptosis.Breast Cancer Res. 2008;10(6):R104. doi: 10.1186/bcr2208. Epub 2008 Dec 5. Breast Cancer Res. 2008. PMID: 19061505 Free PMC article.
-
Transcription Factor MYB as Therapeutic Target: Current Developments.Int J Mol Sci. 2024 Mar 12;25(6):3231. doi: 10.3390/ijms25063231. Int J Mol Sci. 2024. PMID: 38542205 Free PMC article. Review.
Cited by
-
Regulation of the human catalytic subunit of telomerase (hTERT).Gene. 2012 May 1;498(2):135-46. doi: 10.1016/j.gene.2012.01.095. Epub 2012 Feb 13. Gene. 2012. PMID: 22381618 Free PMC article. Review.
-
Deep sequencing and in silico analyses identify MYB-regulated gene networks and signaling pathways in pancreatic cancer.Sci Rep. 2016 Jun 29;6:28446. doi: 10.1038/srep28446. Sci Rep. 2016. PMID: 27354262 Free PMC article.
-
Integrated genome-wide chromatin occupancy and expression analyses identify key myeloid pro-differentiation transcription factors repressed by Myb.Nucleic Acids Res. 2011 Jun;39(11):4664-79. doi: 10.1093/nar/gkr024. Epub 2011 Feb 11. Nucleic Acids Res. 2011. PMID: 21317192 Free PMC article.
-
CDK9 inhibitors selectively target estrogen receptor-positive breast cancer cells through combined inhibition of MYB and MCL-1 expression.Oncotarget. 2016 Feb 23;7(8):9069-83. doi: 10.18632/oncotarget.6997. Oncotarget. 2016. PMID: 26812885 Free PMC article.
-
Signalling mechanisms regulating phenotypic changes in breast cancer cells.Biosci Rep. 2015 Mar 18;35(2):e00178. doi: 10.1042/BSR20140172. Biosci Rep. 2015. PMID: 25643809 Free PMC article.
References
-
- Mucenski M, McLain K, Kier A, Swerdlow S, Schreiner C, Miller T, Pietryga D, Scott WJ, Potter S. A functional c-myb gene is required for normal murine fetal hepatic hematopoiesis. Cell. 1991;65:677–689. - PubMed
-
- Gonda T, Metcalf D. Expression of myb, myc and fos proto-oncogenes during the differentiation of a murine myeloid leukaemia. Nature. 1984;310:249–251. - PubMed
-
- Wolff L. Myb-induced transformation. Crit Rev Oncog. 1996;7:245–260. - PubMed
-
- Ganter B, Lipsick J. Myb and oncogenesis. Adv Cancer Res. 1999;76:21–60. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

