Efficient, high-throughput transfection of human embryonic stem cells
- PMID: 20659329
- PMCID: PMC2941115
- DOI: 10.1186/scrt23
Efficient, high-throughput transfection of human embryonic stem cells
Abstract
Introduction: Genetic manipulation of human embryonic stem cells (hESC) has been limited by their general resistance to common methods used to introduce exogenous DNA or RNA. Efficient and high throughput transfection of nucleic acids into hESC would be a valuable experimental tool to manipulate these cells for research and clinical applications.
Methods: We investigated the ability of two commercially available electroporation systems, the Nucleofection® 96-well Shuttle® System from Lonza and the Neon™ Transfection System from Invitrogen to efficiently transfect hESC. Transfection efficiency was measured by flow cytometry for the expression of the green fluorescent protein and the viability of the transfected cells was determined by an ATP catalyzed luciferase reaction. The transfected cells were also analyzed by flow cytometry for common markers of pluripotency.
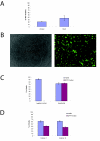
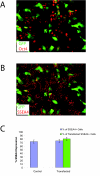
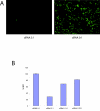
Results: Both systems are capable of transfecting hESC at high efficiencies with little loss of cell viability. However, the reproducibility and the ease of scaling for high throughput applications led us to perform more comprehensive tests on the Nucleofection® 96-well Shuttle® System. We demonstrate that this method yields a large fraction of transiently transfected cells with minimal loss of cell viability and pluripotency, producing protein expression from plasmid vectors in several different hESC lines. The method scales to a 96-well plate with similar transfection efficiencies at the start and end of the plate. We also investigated the efficiency with which stable transfectants can be generated and recovered under antibiotic selection. Finally, we found that this method is effective in the delivery of short synthetic RNA oligonucleotides (siRNA) into hESC for knockdown of translation activity via RNA interference.
Conclusions: Our results indicate that these electroporation methods provide a reliable, efficient, and high-throughput approach to the genetic manipulation of hESC.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

