NO2 inhalation induces maturation of pulmonary CD11c+ cells that promote antigenspecific CD4+ T cell polarization
- PMID: 20659336
- PMCID: PMC2918560
- DOI: 10.1186/1465-9921-11-102
NO2 inhalation induces maturation of pulmonary CD11c+ cells that promote antigenspecific CD4+ T cell polarization
Abstract
Background: Nitrogen dioxide (NO2) is an air pollutant associated with poor respiratory health, asthma exacerbation, and an increased likelihood of inhalational allergies. NO2 is also produced endogenously in the lung during acute inflammatory responses. NO2 can function as an adjuvant, allowing for allergic sensitization to an innocuous inhaled antigen and the generation of an antigen-specific Th2 immune response manifesting in an allergic asthma phenotype. As CD11c+ antigen presenting cells are considered critical for naïve T cell activation, we investigated the role of CD11c+ cells in NO2-promoted allergic sensitization.
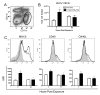
Methods: We systemically depleted CD11c+ cells from transgenic mice expressing a simian diphtheria toxin (DT) receptor under of control of the CD11c promoter by administration of DT. Mice were then exposed to 15 ppm NO2 followed by aerosolized ovalbumin to promote allergic sensitization to ovalbumin and were studied after subsequent inhaled ovalbumin challenges for manifestation of allergic airway disease. In addition, pulmonary CD11c+ cells from wildtype mice were studied after exposure to NO2 and ovalbumin for cellular phenotype by flow cytometry and in vitro cytokine production.
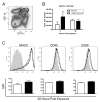
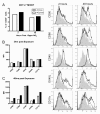
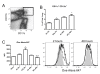
Results: Transient depletion of CD11c+ cells during sensitization attenuated airway eosinophilia during allergen challenge and reduced Th2 and Th17 cytokine production. Lung CD11c+ cells from wildtype mice exhibited a significant increase in MHCII, CD40, and OX40L expression 2 hours following NO2 exposure. By 48 hours, CD11c+MHCII+ DCs within the mediastinal lymph node (MLN) expressed maturation markers, including CD80, CD86, and OX40L. CD11c+CD11b- and CD11c+CD11b+ pulmonary cells exposed to NO2 in vivo increased uptake of antigen 2 hours post exposure, with increased ova-Alexa 647+ CD11c+MHCII+ DCs present in MLN from NO2-exposed mice by 48 hours. Co-cultures of ova-specific CD4+ T cells from naïve mice and CD11c+ pulmonary cells from NO2-exposed mice produced IL-1, IL-12p70, and IL-6 in vitro and augmented antigen-induced IL-5 production.
Conclusions: CD11c+ cells are critical for NO2-promoted allergic sensitization. NO2 exposure causes pulmonary CD11c+ cells to acquire a phenotype capable of increased antigen uptake, migration to the draining lymph node, expression of MHCII and co-stimulatory molecules required to activate naïve T cells, and secretion of polarizing cytokines to shape a Th2/Th17 response.
Figures




Similar articles
-
Interleukin-1 receptor and caspase-1 are required for the Th17 response in nitrogen dioxide-promoted allergic airway disease.Am J Respir Cell Mol Biol. 2013 May;48(5):655-64. doi: 10.1165/rcmb.2012-0423OC. Am J Respir Cell Mol Biol. 2013. PMID: 23371061 Free PMC article.
-
The inhibitory effects of intravenous administration of rabbit immunoglobulin G on airway inflammation are dependent upon Fcγ receptor IIb on CD11c(+) dendritic cells in a murine model.Clin Exp Immunol. 2010 Nov;162(2):315-24. doi: 10.1111/j.1365-2249.2010.04243.x. Epub 2010 Sep 1. Clin Exp Immunol. 2010. PMID: 20819092 Free PMC article.
-
Airway epithelial NF-κB activation promotes allergic sensitization to an innocuous inhaled antigen.Am J Respir Cell Mol Biol. 2011 May;44(5):631-8. doi: 10.1165/rcmb.2010-0106OC. Epub 2010 Jun 25. Am J Respir Cell Mol Biol. 2011. PMID: 20581095 Free PMC article.
-
Complement regulates inhalation tolerance at the dendritic cell/T cell interface.Mol Immunol. 2007 Jan;44(1-3):44-56. doi: 10.1016/j.molimm.2006.06.016. Epub 2006 Aug 4. Mol Immunol. 2007. PMID: 16889830 Review.
-
Epithelial, dendritic, and CD4(+) T cell regulation of and by reactive oxygen and nitrogen species in allergic sensitization.Biochim Biophys Acta. 2011 Nov;1810(11):1025-34. doi: 10.1016/j.bbagen.2011.03.005. Epub 2011 Mar 21. Biochim Biophys Acta. 2011. PMID: 21397661 Free PMC article. Review.
Cited by
-
Airway epithelial regulation of allergic sensitization in asthma.Pulm Pharmacol Ther. 2012 Dec;25(6):438-46. doi: 10.1016/j.pupt.2012.04.005. Epub 2012 May 9. Pulm Pharmacol Ther. 2012. PMID: 22579987 Free PMC article. Review.
-
Interleukin-1 receptor and caspase-1 are required for the Th17 response in nitrogen dioxide-promoted allergic airway disease.Am J Respir Cell Mol Biol. 2013 May;48(5):655-64. doi: 10.1165/rcmb.2012-0423OC. Am J Respir Cell Mol Biol. 2013. PMID: 23371061 Free PMC article.
-
Cockroach allergen Bla g 7 promotes TIM4 expression in dendritic cells leading to Th2 polarization.Mediators Inflamm. 2013;2013:983149. doi: 10.1155/2013/983149. Epub 2013 Sep 24. Mediators Inflamm. 2013. PMID: 24204099 Free PMC article.
-
The Effects of Indoor Pollutants Exposure on Allergy and Lung Inflammation: An Activation State of Neutrophils and Eosinophils in Sputum.Int J Environ Res Public Health. 2020 Jul 28;17(15):5413. doi: 10.3390/ijerph17155413. Int J Environ Res Public Health. 2020. PMID: 32731346 Free PMC article.
-
The endogenous Th17 response in NO2-promoted allergic airway disease is dispensable for airway hyperresponsiveness and distinct from Th17 adoptive transfer.PLoS One. 2013 Sep 19;8(9):e74730. doi: 10.1371/journal.pone.0074730. eCollection 2013. PLoS One. 2013. PMID: 24069338 Free PMC article.
References
-
- Mannino DM, Homa DM, Pertowski CA, Ashizawa A, Nixon LL, Johnson CA, Ball LB, Jack E, Kang DS. Surveillance for asthma--United States, 1960-1995. MMWR CDC Surveill Summ. 1998;47(1):1–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

