The C. elegans adult male germline: stem cells and sexual dimorphism
- PMID: 20659446
- PMCID: PMC2945412
- DOI: 10.1016/j.ydbio.2010.07.022
The C. elegans adult male germline: stem cells and sexual dimorphism
Abstract
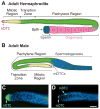
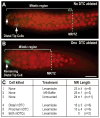
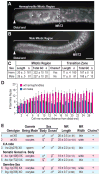
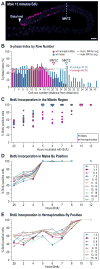
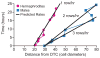
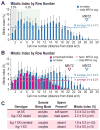
The hermaphrodite Caenorhabditis elegans germline has become a classic model for stem cell regulation, but the male C. elegans germline has been largely neglected. This work provides a cellular analysis of the adult C. elegans male germline, focusing on its predicted stem cell region in the distal gonad. The goals of this study were two-fold: to establish the C. elegans male germline as a stem cell model and to identify sex-specific traits of potential relevance to the sperm/oocyte decision. Our results support two major conclusions. First, adult males do indeed possess a population of germline stem cells (GSCs) with properties similar to those of hermaphrodite GSCs (lack of cell cycle quiescence and lack of reproducibly oriented divisions). Second, germ cells in the mitotic region, including those most distal within the niche, exhibit sex-specific behaviors (e.g. cell cycle length) and therefore have acquired sexual identity. Previous studies demonstrated that some germ cells are not committed to a sperm or oocyte cell fate, even in adults. We propose that germ cells can acquire sexual identity without being committed to a sperm or oocyte cell fate.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures


References
-
- Aherne WA, Camplejohn RS, Wright NA. An Introduction to Cell Population Kinetics. Edward Arnold Ltd; London: 1977.
-
- Angelo G, Van Gilst M. Starvation protects germline stem cells and extends reproductive longevity in C. elegans. Science. 2009;326:954–958. - PubMed
-
- Austin J, Kimble J. glp-1 is required in the germ line for regulation of the decision between mitosis and meiosis in C. elegans. Cell. 1987;51:589–599. - PubMed
-
- Baltus AE, Menke DB, Hu YC, Goodheart ML, Carpenter AE, de Rooij DG, Page DC. In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat Genet. 2006;38:1430–4. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

