Division of labor: subsets of dorsal-appendage-forming cells control the shape of the entire tube
- PMID: 20659448
- PMCID: PMC3153497
- DOI: 10.1016/j.ydbio.2010.07.018
Division of labor: subsets of dorsal-appendage-forming cells control the shape of the entire tube
Abstract
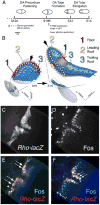
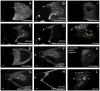
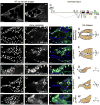
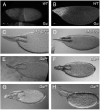
The function of an organ relies on its form, which in turn depends on the individual shapes of the cells that create it and the interactions between them. Despite remarkable progress in the field of developmental biology, how cells collaborate to make a tissue remains an unsolved mystery. To investigate the mechanisms that determine organ structure, we are studying the cells that form the dorsal appendages (DAs) of the Drosophila melanogaster eggshell. These cells consist of two differentially patterned subtypes: roof cells, which form the outward-facing roof of the lumen, and floor cells, which dive underneath the roof cells to seal off the floor of the tube. In this paper, we present three lines of evidence that reveal a further stratification of the DA-forming epithelium. Laser ablation of only a few cells in the anterior of the region causes a disproportionately severe shortening of the appendage. Genetic alteration through the twin peaks allele of tramtrack69 (ttk(twk)), a female-sterile mutation that leads to severely shortened DAs, causes no such shortening when removed from a majority of the DA-forming cells, but rather, produces short appendages only when removed from cells in the very anterior of the tube-forming tissue. Additionally we show that heterotrimeric G-protein function is required for DA morphogenesis. Like TTK69, Gbeta 13F is not required in all DA-forming follicle cells but only in the floor and leading roof cells. The different phenotypes that result from removal of Gbeta 13F from each region demonstrate a striking division of function between different DA-forming cells. Gbeta mutant floor cells are unable to control the width of the appendage while Gbeta mutant leading roof cells fail to direct the elongation of the appendage and the convergent-extension of the roof-cell population.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Following the 'tracks': Tramtrack69 regulates epithelial tube expansion in the Drosophila ovary through Paxillin, Dynamin, and the homeobox protein Mirror.Dev Biol. 2013 Jun 15;378(2):154-69. doi: 10.1016/j.ydbio.2013.03.017. Epub 2013 Mar 30. Dev Biol. 2013. PMID: 23545328 Free PMC article.
-
bullwinkle is required for epithelial morphogenesis during Drosophila oogenesis.Dev Biol. 2004 Mar 15;267(2):320-41. doi: 10.1016/j.ydbio.2003.10.020. Dev Biol. 2004. PMID: 15013797
-
The Drosophila female sterile mutation twin peaks is a novel allele of tramtrack and reveals a requirement for Ttk69 in epithelial morphogenesis.Dev Biol. 2003 Jan 1;253(1):18-35. doi: 10.1006/dbio.2002.0856. Dev Biol. 2003. PMID: 12490195
-
Integration of epithelial patterning and morphogenesis in Drosophila ovarian follicle cells.Dev Dyn. 2000 May;218(1):80-93. doi: 10.1002/(SICI)1097-0177(200005)218:1<80::AID-DVDY7>3.0.CO;2-8. Dev Dyn. 2000. PMID: 10822261 Review.
-
Tube formation in Drosophila egg chambers.Tissue Eng Part A. 2008 Sep;14(9):1479-88. doi: 10.1089/ten.tea.2008.0124. Tissue Eng Part A. 2008. PMID: 18707228 Free PMC article. Review.
Cited by
-
Pattern formation by a moving morphogen source.Phys Biol. 2011 Aug;8(4):045003. doi: 10.1088/1478-3975/8/4/045003. Epub 2011 Jul 12. Phys Biol. 2011. PMID: 21750363 Free PMC article.
-
Detecting New Allies: Modifier Screen Identifies a Genetic Interaction Between Imaginal disc growth factor 3 and combover, a Rho-kinase Substrate, During Dorsal Appendage Tube Formation in Drosophila.G3 (Bethesda). 2020 Oct 5;10(10):3585-3599. doi: 10.1534/g3.120.401476. G3 (Bethesda). 2020. PMID: 32855169 Free PMC article.
-
Following the 'tracks': Tramtrack69 regulates epithelial tube expansion in the Drosophila ovary through Paxillin, Dynamin, and the homeobox protein Mirror.Dev Biol. 2013 Jun 15;378(2):154-69. doi: 10.1016/j.ydbio.2013.03.017. Epub 2013 Mar 30. Dev Biol. 2013. PMID: 23545328 Free PMC article.
-
A discrete model of Drosophila eggshell patterning reveals cell-autonomous and juxtacrine effects.PLoS Comput Biol. 2014 Mar 27;10(3):e1003527. doi: 10.1371/journal.pcbi.1003527. eCollection 2014 Mar. PLoS Comput Biol. 2014. PMID: 24675973 Free PMC article.
-
Intercellular protein movement in syncytial Drosophila follicle cells.J Cell Sci. 2011 Dec 1;124(Pt 23):4077-86. doi: 10.1242/jcs.090456. Epub 2011 Dec 1. J Cell Sci. 2011. PMID: 22135360 Free PMC article.
References
-
- Althauser C, Jordan KC, Deng WM, Ruohola-Baker H. Fringe-dependent Notch activation and Tramtrack function are required for specification of the polar cells in Drosophila oogenesis. Dev Dyn. 2005;232:1013–1020. - PubMed
-
- Bloor JW, Kiehart DP. zipper nonmuscle myosin-II functions downstream of PS2 integrin in Drosophila myogenesis and is necessary for myofibril formation. Dev Biol. 2001;239:215–228. - PubMed
-
- Caussinus E, Colombelli J, Affolter M. Tip-cell migration controls stalk-cell intercalation during Drosophila tracheal tube elongation. Curr Biol. 2008;18:1727–1734. - PubMed
-
- Chen Y, Schüpbach T. The role of brinker in eggshell patterning. Mech Dev. 2006;123:395–406. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

