Membrane docking geometry and target lipid stoichiometry of membrane-bound PKCα C2 domain: a combined molecular dynamics and experimental study
- PMID: 20659476
- PMCID: PMC3602446
- DOI: 10.1016/j.jmb.2010.07.037
Membrane docking geometry and target lipid stoichiometry of membrane-bound PKCα C2 domain: a combined molecular dynamics and experimental study
Abstract
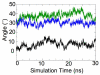
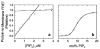
Protein kinase Cα (PKCα) possesses a conserved C2 domain (PKCα C2 domain) that acts as a Ca(2+)-regulated membrane targeting element. Upon activation by Ca(2+), the PKCα C2 domain directs the kinase protein to the plasma membrane, thereby stimulating an array of cellular pathways. At sufficiently high Ca(2+) concentrations, binding of the C2 domain to the target lipid phosphatidylserine (PS) is sufficient to drive membrane association; however, at typical physiological Ca(2+) concentrations, binding to both PS and phosphoinositidyl-4,5-bisphosphate (PIP(2)) is required for specific plasma membrane targeting. Recent EPR studies have revealed the membrane docking geometries of the PKCα C2 domain docked to (i) PS alone and (ii) both PS and PIP(2) simultaneously. These two EPR docking geometries exhibit significantly different tilt angles relative to the plane of the membrane, presumably induced by the large size of the PIP(2) headgroup. The present study utilizes the two EPR docking geometries as starting points for molecular dynamics simulations that investigate atomic features of the protein-membrane interaction. The simulations yield approximately the same PIP(2)-triggered change in tilt angle observed by EPR. Moreover, the simulations predict a PIP(2):C2 stoichiometry approaching 2:1 at a high PIP(2) mole density. Direct binding measurements titrating the C2 domain with PIP(2) in lipid bilayers yield a 1:1 stoichiometry at moderate mole densities and a saturating 2:1 stoichiometry at high PIP(2) mole densities. Thus, the experiment confirms the target lipid stoichiometry predicted by EPR-guided molecular dynamics simulations. Potential biological implications of the observed docking geometries and PIP(2) stoichiometries are discussed.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Effect of PIP2 binding on the membrane docking geometry of PKC alpha C2 domain: an EPR site-directed spin-labeling and relaxation study.Biochemistry. 2008 Aug 12;47(32):8301-16. doi: 10.1021/bi800711t. Epub 2008 Jul 9. Biochemistry. 2008. PMID: 18610985 Free PMC article.
-
Differential roles of phosphatidylserine, PtdIns(4,5)P2, and PtdIns(3,4,5)P3 in plasma membrane targeting of C2 domains. Molecular dynamics simulation, membrane binding, and cell translocation studies of the PKCalpha C2 domain.J Biol Chem. 2008 Sep 19;283(38):26047-58. doi: 10.1074/jbc.M802617200. Epub 2008 Jul 11. J Biol Chem. 2008. PMID: 18621733 Free PMC article.
-
Mechanism of specific membrane targeting by C2 domains: localized pools of target lipids enhance Ca2+ affinity.Biochemistry. 2007 Apr 10;46(14):4322-36. doi: 10.1021/bi062140c. Epub 2007 Mar 17. Biochemistry. 2007. PMID: 17367165 Free PMC article.
-
The C2 domains of classical/conventional PKCs are specific PtdIns(4,5)P(2)-sensing domains.Biochem Soc Trans. 2007 Nov;35(Pt 5):1046-8. doi: 10.1042/BST0351046. Biochem Soc Trans. 2007. PMID: 17956275 Review.
-
Use of EPR power saturation to analyze the membrane-docking geometries of peripheral proteins: applications to C2 domains.Annu Rev Biophys Biomol Struct. 2005;34:71-90. doi: 10.1146/annurev.biophys.34.040204.144534. Annu Rev Biophys Biomol Struct. 2005. PMID: 15869384 Free PMC article. Review.
Cited by
-
Lateral diffusion of peripheral membrane proteins on supported lipid bilayers is controlled by the additive frictional drags of (1) bound lipids and (2) protein domains penetrating into the bilayer hydrocarbon core.Chem Phys Lipids. 2013 Jul-Aug;172-173:67-77. doi: 10.1016/j.chemphyslip.2013.04.005. Epub 2013 May 20. Chem Phys Lipids. 2013. PMID: 23701821 Free PMC article.
-
Regulation of PI3K by PKC and MARCKS: Single-Molecule Analysis of a Reconstituted Signaling Pathway.Biophys J. 2016 Apr 26;110(8):1811-1825. doi: 10.1016/j.bpj.2016.03.001. Biophys J. 2016. PMID: 27119641 Free PMC article.
-
A Conserved Electrostatic Membrane-Binding Surface in Synaptotagmin-Like Proteins Revealed Using Molecular Phylogenetic Analysis and Homology Modeling.bioRxiv [Preprint]. 2023 Oct 29:2023.07.13.548768. doi: 10.1101/2023.07.13.548768. bioRxiv. 2023. Update in: Protein Sci. 2024 Jan;33(1):e4850. doi: 10.1002/pro.4850. PMID: 37502952 Free PMC article. Updated. Preprint.
-
Biophysical and computational studies of membrane penetration by the GRP1 pleckstrin homology domain.Structure. 2011 Sep 7;19(9):1338-46. doi: 10.1016/j.str.2011.04.010. Structure. 2011. PMID: 21893292 Free PMC article.
-
Investigation of the Effect of Bilayer Composition on PKCα-C2 Domain Docking Using Molecular Dynamics Simulations.J Phys Chem B. 2017 Jan 12;121(1):78-88. doi: 10.1021/acs.jpcb.6b10188. Epub 2016 Dec 20. J Phys Chem B. 2017. PMID: 27997184 Free PMC article.
References
-
- Lemmon MA. Membrane recognition by phospholipid-binding domains. Nat. Rev. Mol. Cell. Biol. 2008;9:99–111. - PubMed
-
- Corbalan-Garcia S, Guerrero-Valero M, Marin-Vicente C, Gomez-Fernandez JC. The C2 domains of classical/conventional PKCs are specific PtdIns(4,5)P2-sensing domains. Biochem. Soc. Trans. 2007;35:1046–1048. - PubMed
-
- Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–496. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

