A protein-based smallpox vaccine protects non-human primates from a lethal monkeypox virus challenge
- PMID: 20659519
- PMCID: PMC2939220
- DOI: 10.1016/j.vaccine.2010.07.030
A protein-based smallpox vaccine protects non-human primates from a lethal monkeypox virus challenge
Abstract
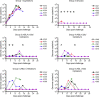
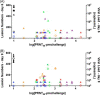
Concerns about infections caused by orthopoxviruses, such as variola and monkeypox viruses, drive ongoing efforts to develop novel smallpox vaccines that are both effective and safe to use in diverse populations. A subunit smallpox vaccine comprising vaccinia virus membrane proteins A33, B5, L1, A27 and aluminum hydroxide (alum) ± CpG was administered to non-human primates, which were subsequently challenged with a lethal intravenous dose of monkeypox virus. Alum adjuvanted vaccines provided only partial protection but the addition of CpG provided full protection that was associated with a more homogeneous antibody response and stronger IgG1 responses. These results indicate that it is feasible to develop a highly effective subunit vaccine against orthopoxvirus infections as a safer alternative to live vaccinia virus vaccination.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Short-term and longer-term protective immune responses generated by subunit vaccination with smallpox A33, B5, L1 or A27 proteins adjuvanted with aluminum hydroxide and CpG in mice challenged with vaccinia virus.Vaccine. 2020 Aug 27;38(38):6007-6018. doi: 10.1016/j.vaccine.2020.07.018. Epub 2020 Jul 30. Vaccine. 2020. PMID: 32741672 Free PMC article.
-
Adjuvant-enhanced antibody responses to recombinant proteins correlates with protection of mice and monkeys to orthopoxvirus challenges.Vaccine. 2007 Apr 12;25(15):2787-99. doi: 10.1016/j.vaccine.2006.12.037. Epub 2007 Jan 3. Vaccine. 2007. PMID: 17229505 Free PMC article.
-
Different immunogens and prime-boost vaccination strategies affect the efficacy of recombinant candidate vaccines against pathogenic orthopoxviruses.Virol J. 2024 Nov 7;21(1):282. doi: 10.1186/s12985-024-02534-4. Virol J. 2024. PMID: 39511612 Free PMC article.
-
Poxvirus Vaccines: Past, Present, and Future.Adv Exp Med Biol. 2024;1451:273-287. doi: 10.1007/978-3-031-57165-7_17. Adv Exp Med Biol. 2024. PMID: 38801584 Review.
-
Third-generation smallpox vaccines: challenges in the absence of clinical smallpox.Future Microbiol. 2010 Sep;5(9):1367-82. doi: 10.2217/fmb.10.98. Future Microbiol. 2010. PMID: 20860482 Review.
Cited by
-
A vaccinia virus renaissance: new vaccine and immunotherapeutic uses after smallpox eradication.Hum Vaccin Immunother. 2012 Jul;8(7):961-70. doi: 10.4161/hv.21080. Epub 2012 Jul 1. Hum Vaccin Immunother. 2012. PMID: 22777090 Free PMC article. Review.
-
Short-term and longer-term protective immune responses generated by subunit vaccination with smallpox A33, B5, L1 or A27 proteins adjuvanted with aluminum hydroxide and CpG in mice challenged with vaccinia virus.Vaccine. 2020 Aug 27;38(38):6007-6018. doi: 10.1016/j.vaccine.2020.07.018. Epub 2020 Jul 30. Vaccine. 2020. PMID: 32741672 Free PMC article.
-
TLR3 and TLR9 agonists improve postexposure vaccination efficacy of live smallpox vaccines.PLoS One. 2014 Oct 28;9(10):e110545. doi: 10.1371/journal.pone.0110545. eCollection 2014. PLoS One. 2014. PMID: 25350003 Free PMC article.
-
Prevention and Treatment of Monkeypox: A Systematic Review of Preclinical Studies.Viruses. 2022 Nov 11;14(11):2496. doi: 10.3390/v14112496. Viruses. 2022. PMID: 36423105 Free PMC article.
-
Side-by-side comparison of gene-based smallpox vaccine with MVA in nonhuman primates.PLoS One. 2012;7(7):e42353. doi: 10.1371/journal.pone.0042353. Epub 2012 Jul 31. PLoS One. 2012. PMID: 22860117 Free PMC article.
References
-
- Fenner F., Henderson D.A., Arita I., Jezek Z., Ladnyi ID . 1st ed. World Health Organization; Geneva: 1988. Smallpox and its eradication.
-
- CDC Notice to readers: newly licensed smallpox vaccine to replace old smallpox vaccine. MMWR Morb Mortal Wkly Rep. 2008;57(8):207–208.
-
- Poland G.A. Smallpox vaccines: from first to second to third generation. Lancet. 2005;365(9457):362–363. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

