Similarities in the behavior and molecular deficits in the frontal cortex between the neurotensin receptor subtype 1 knockout mice and chronic phencyclidine-treated mice: relevance to schizophrenia
- PMID: 20659557
- PMCID: PMC2939306
- DOI: 10.1016/j.nbd.2010.07.011
Similarities in the behavior and molecular deficits in the frontal cortex between the neurotensin receptor subtype 1 knockout mice and chronic phencyclidine-treated mice: relevance to schizophrenia
Abstract
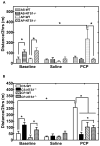
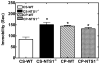
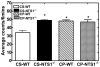
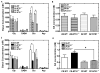
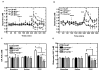
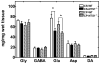
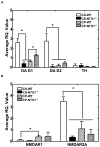
Much evidence suggests that targeting the neurotensin (NT) system may provide a novel and promising treatment for schizophrenia. Our recent work shows that: NTS1 knockout (NTS1(-/-)) mice may provide a potential animal model for studying schizophrenia by investigating the effect of deletion NTS1 receptor on amphetamine-induced hyperactivity and neurochemical changes. The data indicate a hyper-dopaminergic state similar to the excessive striatal DA activity reported in schizophrenia. The present study was done to determine if NTS1(-/-) mice also have similar changes in behavior, in prefrontal neurotransmitters, and in protein expression, as observed in wild type (WT) mice treated with the psychotomimetic phencylclidine (PCP), an animal model for schizophrenia. Our results showed many similarities between untreated NTS1(-/-) mice and WT mice chronically treated with PCP (as compared with untreated WT mice): 1) lower PCP-induced locomotor activity; 2) similar avolition-like behavior in forced-swim test and tail suspension test; 3) lower prefrontal glutamate levels; 4) less PCP-induced dopamine release in medial prefrontal cortex (mPFC); and 5) down-regulation of mRNA and protein for DA D(1), DA D(2), and NMDAR2A in mPFC. Therefore, these data strengthen the hypothesis that the NTS1(-/-) mouse is an animal model of schizophrenia, particularly for the dysfunction of the prefrontal cortex. In addition, after chronic PCP administration, the DA D(1) receptor was up-regulated in NTS1(-/-) mice, results which suggest a possible interaction of NTS1/DA D(1) in mPFC contributing to chronic PCP-induced schizophrenia-like signs.
Published by Elsevier Inc.
Figures

Similar articles
-
The novel neurotensin analog NT69L blocks phencyclidine (PCP)-induced increases in locomotor activity and PCP-induced increases in monoamine and amino acids levels in the medial prefrontal cortex.Brain Res. 2010 Jan 22;1311:28-36. doi: 10.1016/j.brainres.2009.11.048. Epub 2009 Nov 27. Brain Res. 2010. PMID: 19948149 Free PMC article.
-
Effect of amphetamine on extracellular concentrations of amino acids in striatum in neurotensin subtype 1 and 2 receptor null mice: a possible interaction between neurotensin receptors and amino acid systems for study of schizophrenia.Neuropharmacology. 2010 Jun;58(7):1174-8. doi: 10.1016/j.neuropharm.2010.02.016. Epub 2010 Mar 1. Neuropharmacology. 2010. PMID: 20193696 Free PMC article.
-
Effects of D-amphetamine and phencyclidine on behavior and extracellular concentrations of neurotensin and dopamine in the ventral striatum and the medial prefrontal cortex of the rat.Behav Brain Res. 1995 Dec 14;72(1-2):103-14. doi: 10.1016/0166-4328(96)00138-6. Behav Brain Res. 1995. PMID: 8788863
-
Animal model of schizophrenia: dysfunction of NMDA receptor-signaling in mice following withdrawal from repeated administration of phencyclidine.Ann N Y Acad Sci. 2006 Nov;1086:160-8. doi: 10.1196/annals.1377.003. Ann N Y Acad Sci. 2006. PMID: 17185514 Review.
-
Phencyclidine and genetic animal models of schizophrenia developed in relation to the glutamate hypothesis.Methods Find Exp Clin Pharmacol. 2007 May;29(4):291-301. doi: 10.1358/mf.2007.29.4.1075358. Methods Find Exp Clin Pharmacol. 2007. PMID: 17609743 Review.
Cited by
-
Neurotensin and Neurotensin Receptors in Stress-related Disorders: Pathophysiology & Novel Drug Targets.Curr Neuropharmacol. 2024;22(5):916-934. doi: 10.2174/1570159X21666230803101629. Curr Neuropharmacol. 2024. PMID: 37534788 Free PMC article. Review.
-
Antioxidant treatment strategies for hyperphenylalaninemia.Metab Brain Dis. 2013 Dec;28(4):541-50. doi: 10.1007/s11011-013-9414-2. Epub 2013 May 9. Metab Brain Dis. 2013. PMID: 23657560 Review.
-
Astrocyte Activation, but not Microglia, Is Associated with the Experimental Mouse Model of Schizophrenia Induced by Chronic Ketamine.J Mol Neurosci. 2022 Sep;72(9):1902-1915. doi: 10.1007/s12031-022-02046-2. Epub 2022 Jul 8. J Mol Neurosci. 2022. PMID: 35802289
-
Bee Pollen and Probiotics May Alter Brain Neuropeptide Levels in a Rodent Model of Autism Spectrum Disorders.Metabolites. 2022 Jun 18;12(6):562. doi: 10.3390/metabo12060562. Metabolites. 2022. PMID: 35736494 Free PMC article.
-
Disruption of medial prefrontal synchrony in the subchronic phencyclidine model of schizophrenia in rats.Neuroscience. 2015 Feb 26;287:157-63. doi: 10.1016/j.neuroscience.2014.12.014. Epub 2014 Dec 24. Neuroscience. 2015. PMID: 25542422 Free PMC article.
References
-
- Abdel-Naby Sayed M, et al. Enhancement of immobility induced by repeated phencyclidine injection: association with c-Fos protein in the mouse brain. Behav Brain Res. 2001;124:71–6. - PubMed
-
- Allen RM, Young SJ. Phencyclidine-induced psychosis. Am J Psychiatry. 1978;135:1081–4. - PubMed
-
- Antonelli T, et al. Neurotensin receptor mechanisms and its modulation of glutamate transmission in the brain: relevance for neurodegenerative diseases and their treatment. Prog Neurobiol. 2007;83:92–109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

