NMR solution structure and biophysical characterization of Vibrio harveyi acyl carrier protein A75H: effects of divalent metal ions
- PMID: 20659901
- PMCID: PMC2945550
- DOI: 10.1074/jbc.M110.128298
NMR solution structure and biophysical characterization of Vibrio harveyi acyl carrier protein A75H: effects of divalent metal ions
Abstract
Bacterial acyl carrier protein (ACP) is a highly anionic, 9 kDa protein that functions as a cofactor protein in fatty acid biosynthesis. Escherichia coli ACP is folded at neutral pH and in the absence of divalent cations, while Vibrio harveyi ACP, which is very similar at 86% sequence identity, is unfolded under the same conditions. V. harveyi ACP adopts a folded conformation upon the addition of divalent cations such as Ca(2+) and Mg(2+) and a mutant, A75H, was previously identified that restores the folded conformation at pH 7 in the absence of divalent cations. In this study we sought to understand the unique folding behavior of V. harveyi ACP using NMR spectroscopy and biophysical methods. The NMR solution structure of V. harveyi ACP A75H displays the canonical ACP structure with four helices surrounding a hydrophobic core, with a narrow pocket closed off from the solvent to house the acyl chain. His-75, which is charged at neutral pH, participates in a stacking interaction with Tyr-71 in the far C-terminal end of helix IV. pH titrations and the electrostatic profile of ACP suggest that V. harveyi ACP is destabilized by anionic charge repulsion around helix II that can be partially neutralized by His-75 and is further reduced by divalent cation binding. This is supported by differential scanning calorimetry data which indicate that calcium binding further increases the melting temperature of V. harveyi ACP A75H by ∼20 °C. Divalent cation binding does not alter ACP dynamics on the ps-ns timescale as determined by (15)N NMR relaxation experiments, however, it clearly stabilizes the protein fold as observed by hydrogen-deuterium exchange studies. Finally, we demonstrate that the E. coli ACP H75A mutant is similarly unfolded as wild-type V. harveyi ACP, further stressing the importance of this particular residue for proper protein folding.
Figures

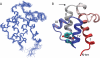


Similar articles
-
Neutralization of acidic residues in helix II stabilizes the folded conformation of acyl carrier protein and variably alters its function with different enzymes.J Biol Chem. 2007 Feb 16;282(7):4494-4503. doi: 10.1074/jbc.M608234200. Epub 2006 Dec 18. J Biol Chem. 2007. PMID: 17179150
-
Identification of a key residue in the conformational stability of acyl carrier protein.Biochim Biophys Acta. 2002 Dec 16;1601(2):208-14. doi: 10.1016/s1570-9639(02)00470-3. Biochim Biophys Acta. 2002. PMID: 12445484
-
Hydrodynamic properties of Vibrio harveyi acyl carrier protein and its fatty-acylated derivatives.Arch Biochem Biophys. 1997 Aug 1;344(1):159-64. doi: 10.1006/abbi.1997.0203. Arch Biochem Biophys. 1997. PMID: 9244393
-
Acyl carrier protein: structure-function relationships in a conserved multifunctional protein family.Biochem Cell Biol. 2007 Dec;85(6):649-62. doi: 10.1139/o07-109. Biochem Cell Biol. 2007. PMID: 18059524 Review.
-
A Divalent Metal Cation-Metabolite Interaction Model Reveals Cation Buffering and Speciation.Biochemistry. 2024 Jul 16;63(14):1709-1717. doi: 10.1021/acs.biochem.4c00125. Epub 2024 Jul 8. Biochemistry. 2024. PMID: 38975737 Review.
Cited by
-
Modular type I polyketide synthase acyl carrier protein domains share a common N-terminally extended fold.Sci Rep. 2019 Feb 20;9(1):2325. doi: 10.1038/s41598-019-38747-9. Sci Rep. 2019. PMID: 30787330 Free PMC article.
-
Rigidifying acyl carrier protein domain in iterative type I PKS CalE8 does not affect its function.Biophys J. 2012 Sep 5;103(5):1037-44. doi: 10.1016/j.bpj.2012.08.006. Biophys J. 2012. PMID: 23009853 Free PMC article.
-
Deciphering the Binding Interactions between Acinetobacter baumannii ACP and β-ketoacyl ACP Synthase III to Improve Antibiotic Targeting Using NMR Spectroscopy.Int J Mol Sci. 2021 Mar 24;22(7):3317. doi: 10.3390/ijms22073317. Int J Mol Sci. 2021. PMID: 33805050 Free PMC article.
-
Fatty acid biosynthesis revisited: structure elucidation and metabolic engineering.Mol Biosyst. 2015 Jan;11(1):38-59. doi: 10.1039/c4mb00443d. Epub 2014 Oct 31. Mol Biosyst. 2015. PMID: 25360565 Free PMC article. Review.
-
Structural and dynamic characterization of a freestanding acyl carrier protein involved in the biosynthesis of cyclic lipopeptide antibiotics.Protein Sci. 2017 May;26(5):946-959. doi: 10.1002/pro.3138. Epub 2017 Mar 1. Protein Sci. 2017. PMID: 28187530 Free PMC article.
References
-
- Maier T., Jenni S., Ban N. (2006) Science 311, 1258–1262 - PubMed
-
- Chan D. I., Vogel H. J. (2010) Biochem. J. 430, 1–19 - PubMed
-
- Byers D. M., Gong H. (2007) Biochem. Cell Biol. 85, 649–662 - PubMed
-
- Zhang Y. M., Rock C. O. (2008) Nat. Rev. Microbiol. 6, 222–233 - PubMed
-
- White S. W., Zheng J., Zhang Y. M., Rock C. O. (2005) Annu. Rev. Biochem. 74, 791–831 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

