Electroclinical characterization of epileptic seizures in leucine-rich, glioma-inactivated 1-deficient mice
- PMID: 20659958
- PMCID: PMC2929330
- DOI: 10.1093/brain/awq171
Electroclinical characterization of epileptic seizures in leucine-rich, glioma-inactivated 1-deficient mice
Abstract
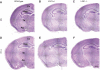
Mutations of the LGI1 (leucine-rich, glioma-inactivated 1) gene underlie autosomal dominant lateral temporal lobe epilepsy, a focal idiopathic inherited epilepsy syndrome. The LGI1 gene encodes a protein secreted by neurons, one of the only non-ion channel genes implicated in idiopathic familial epilepsy. While mutations probably result in a loss of function, the role of LGI1 in the pathophysiology of epilepsy remains unclear. Here we generated a germline knockout mouse for LGI1 and examined spontaneous seizure characteristics, changes in threshold for induced seizures and hippocampal pathology. Frequent spontaneous seizures emerged in homozygous LGI1(-/-) mice during the second postnatal week. Properties of these spontaneous events were examined in a simultaneous video and intracranial electroencephalographic recording. Their mean duration was 120 +/- 12 s, and behavioural correlates consisted of an initial immobility, automatisms, sometimes followed by wild running and tonic and/or clonic movements. Electroencephalographic monitoring indicated that seizures originated earlier in the hippocampus than in the cortex. LGI1(-/-) mice did not survive beyond postnatal day 20, probably due to seizures and failure to feed. While no major developmental abnormalities were observed, after recurrent seizures we detected neuronal loss, mossy fibre sprouting, astrocyte reactivity and granule cell dispersion in the hippocampus of LGI1(-/-) mice. In contrast, heterozygous LGI1(+/-) littermates displayed no spontaneous behavioural epileptic seizures, but auditory stimuli induced seizures at a lower threshold, reflecting the human pathology of sound-triggered seizures in some patients. We conclude that LGI1(+/-) and LGI1(-/-) mice may provide useful models for lateral temporal lobe epilepsy, and more generally idiopathic focal epilepsy.
Figures






References
-
- Baulac S, Baulac M. Advances on the genetics of mendelian idiopathic epilepsies. Neurol Clin. 2009;27:1041–61. - PubMed
-
- Ben-Ari Y, Holmes GL. Effects of seizures on developmental processes in the immature brain. Lancet Neurol. 2006;5:1055–63. - PubMed
-
- Brodtkorb E, Nakken KO, Steinlein OK. No evidence for a seriously increased malignancy risk in LGI1-caused epilepsy. Epilepsy Res. 2003;56:205–8. - PubMed
-
- Chabrol E, Popescu C, Gourfinkel-An I, Trouillard O, Depienne C, Senechal K, et al. Two novel epilepsy-linked mutations leading to a loss of function of LGI1. Arch Neurol. 2007;64:217–22. - PubMed
-
- Chernova OB, Somerville RP, Cowell JK. A novel gene, LGI1, from 10q24 is rearranged and downregulated in malignant brain tumors. Oncogene. 1998;17:2873–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

