Absence of thyroid hormone activation during development underlies a permanent defect in adaptive thermogenesis
- PMID: 20660060
- PMCID: PMC2940501
- DOI: 10.1210/en.2010-0511
Absence of thyroid hormone activation during development underlies a permanent defect in adaptive thermogenesis
Abstract
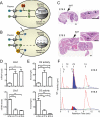
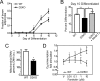
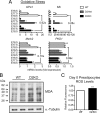
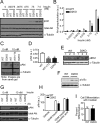
Type 2 deiodinase (D2), which is highly expressed in brown adipose tissue (BAT), is an enzyme that amplifies thyroid hormone signaling in individual cells. Mice with inactivation of the D2 pathway (D2KO) exhibit dramatically impaired thermogenesis in BAT, leading to hypothermia during cold exposure and a greater susceptibility to diet-induced obesity. This was interpreted as a result of defective acute activation of BAT D2. Here we report that the adult D2KO BAT has a permanent thermogenic defect that stems from impaired embryonic BAT development. D2KO embryos have normal serum T3 but due to lack of D2-generated T3 in BAT, this tissue exhibits decreased expression of genes defining BAT identity [i.e. UCP1, PGC-1alpha and Dio2 (nonfunctional)], which results in impaired differentiation and oxidative capacity. Coinciding with a reduction of these T3-responsive genes, there is oxidative stress that in a cell model of brown adipogenesis can be linked to decreased insulin signaling and decreased adipogenesis. This discovery highlights the importance of deiodinase-controlled thyroid hormone signaling in BAT development, where it has important metabolic repercussions for energy homeostasis in adulthood.
Figures

Comment in
-
Type 2 deiodinase and brown fat: the heat is on--or off.Endocrinology. 2010 Sep;151(9):4087-9. doi: 10.1210/en.2010-0712. Endocrinology. 2010. PMID: 20736402 No abstract available.
Similar articles
-
Type 2 deiodinase and brown fat: the heat is on--or off.Endocrinology. 2010 Sep;151(9):4087-9. doi: 10.1210/en.2010-0712. Endocrinology. 2010. PMID: 20736402 No abstract available.
-
Inactivation of Type 3 Deiodinase Results in Life-long Changes in the Brown Adipose Tissue Transcriptome in the Male Mouse.Endocrinology. 2022 May 1;163(5):bqac026. doi: 10.1210/endocr/bqac026. Endocrinology. 2022. PMID: 35238380 Free PMC article.
-
Adaptive activation of thyroid hormone and energy expenditure.Biosci Rep. 2005 Jun-Aug;25(3-4):191-208. doi: 10.1007/s10540-005-2885-6. Biosci Rep. 2005. PMID: 16283553 Review.
-
The role of thyroid hormone and brown adipose tissue in energy homoeostasis.Lancet Diabetes Endocrinol. 2013 Nov;1(3):250-8. doi: 10.1016/S2213-8587(13)70069-X. Epub 2013 Oct 18. Lancet Diabetes Endocrinol. 2013. PMID: 24622373 Free PMC article. Review.
-
Tissue-specific inactivation of type 2 deiodinase reveals multilevel control of fatty acid oxidation by thyroid hormone in the mouse.Diabetes. 2014 May;63(5):1594-604. doi: 10.2337/db13-1768. Epub 2014 Jan 31. Diabetes. 2014. PMID: 24487027 Free PMC article.
Cited by
-
Role of the type 2 iodothyronine deiodinase (D2) in the control of thyroid hormone signaling.Biochim Biophys Acta. 2013 Jul;1830(7):3956-64. doi: 10.1016/j.bbagen.2012.08.019. Epub 2012 Aug 29. Biochim Biophys Acta. 2013. PMID: 22967761 Free PMC article. Review.
-
Type 2 Deiodinase Disruption in Astrocytes Results in Anxiety-Depressive-Like Behavior in Male Mice.Endocrinology. 2016 Sep;157(9):3682-95. doi: 10.1210/en.2016-1272. Epub 2016 Aug 8. Endocrinology. 2016. PMID: 27501182 Free PMC article.
-
Agonist Antibody Converts Stem Cells into Migrating Brown Adipocyte-Like Cells in Heart.Cells. 2020 Jan 20;9(1):256. doi: 10.3390/cells9010256. Cells. 2020. PMID: 31968623 Free PMC article.
-
Thyroid hormone replacement therapy: three 'simple' questions, complex answers.Eur Thyroid J. 2012 Jul;1(2):88-98. doi: 10.1159/000339447. Epub 2012 Jun 27. Eur Thyroid J. 2012. PMID: 24783002 Free PMC article.
-
American Thyroid Association Guide to investigating thyroid hormone economy and action in rodent and cell models.Thyroid. 2014 Jan;24(1):88-168. doi: 10.1089/thy.2013.0109. Epub 2013 Dec 12. Thyroid. 2014. PMID: 24001133 Free PMC article.
References
-
- Nedergaard J, Cannon B 2010 The changed metabolic world with human brown adipose tissue: therapeutic visions. Cell Metab 11:268–272 - PubMed
-
- Bianco AC, Sheng XY, Silva JE 1988 Triiodothyronine amplifies norepinephrine stimulation of uncoupling protein gene transcription by a mechanism not requiring protein synthesis. J Biol Chem 263:18168–18175 - PubMed
-
- Sellers EA, You SS 1950 Role of the thyroid in metabolic responses to a cold environment. Am J Physiol 163:81–91 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

