The BNIP-2 and Cdc42GAP homology (BCH) domain of p50RhoGAP/Cdc42GAP sequesters RhoA from inactivation by the adjacent GTPase-activating protein domain
- PMID: 20660160
- PMCID: PMC2938388
- DOI: 10.1091/mbc.E09-05-0408
The BNIP-2 and Cdc42GAP homology (BCH) domain of p50RhoGAP/Cdc42GAP sequesters RhoA from inactivation by the adjacent GTPase-activating protein domain
Abstract
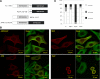
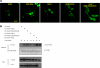
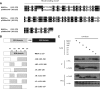
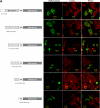
The BNIP-2 and Cdc42GAP homology (BCH) domain is a novel regulator for Rho GTPases, but its impact on p50-Rho GTPase-activating protein (p50RhoGAP or Cdc42GAP) in cells remains elusive. Here we show that deletion of the BCH domain from p50RhoGAP enhanced its GAP activity and caused drastic cell rounding. Introducing constitutively active RhoA or inactivating GAP domain blocked such effect, whereas replacing the BCH domain with endosome-targeting SNX3 excluded requirement of endosomal localization in regulating the GAP activity. Substitution with homologous BCH domain from Schizosaccharomyces pombe, which does not bind mammalian RhoA, also led to complete loss of suppression. Interestingly, the p50RhoGAP BCH domain only targeted RhoA, but not Cdc42 or Rac1, and it was unable to distinguish between GDP and the GTP-bound form of RhoA. Further mutagenesis revealed a RhoA-binding motif (residues 85-120), which when deleted, significantly reduced BCH inhibition on GAP-mediated cell rounding, whereas its full suppression also required an intramolecular interaction motif (residues 169-197). Therefore, BCH domain serves as a local modulator in cis to sequester RhoA from inactivation by the adjacent GAP domain, adding to a new paradigm for regulating p50RhoGAP signaling.
Figures





Similar articles
-
Structural basis for p50RhoGAP BCH domain-mediated regulation of Rho inactivation.Proc Natl Acad Sci U S A. 2021 May 25;118(21):e2014242118. doi: 10.1073/pnas.2014242118. Proc Natl Acad Sci U S A. 2021. PMID: 34006635 Free PMC article.
-
BNIP-Salpha induces cell rounding and apoptosis by displacing p50RhoGAP and facilitating RhoA activation via its unique motifs in the BNIP-2 and Cdc42GAP homology domain.Oncogene. 2006 Apr 13;25(16):2393-408. doi: 10.1038/sj.onc.1209274. Oncogene. 2006. PMID: 16331259
-
Structural basis for the distinct roles of non-conserved Pro116 and conserved Tyr124 of BCH domain of yeast p50RhoGAP.Cell Mol Life Sci. 2024 May 13;81(1):216. doi: 10.1007/s00018-024-05238-8. Cell Mol Life Sci. 2024. PMID: 38740643 Free PMC article.
-
Functional plasticity of the BNIP-2 and Cdc42GAP Homology (BCH) domain in cell signaling and cell dynamics.FEBS Lett. 2012 Aug 14;586(17):2674-91. doi: 10.1016/j.febslet.2012.04.023. Epub 2012 Apr 21. FEBS Lett. 2012. PMID: 22710163 Review.
-
RhoA regulation in space and time.FEBS Lett. 2023 Mar;597(6):836-849. doi: 10.1002/1873-3468.14578. Epub 2023 Jan 29. FEBS Lett. 2023. PMID: 36658753 Review.
Cited by
-
Bmcc1s, a novel brain-isoform of Bmcc1, affects cell morphology by regulating MAP6/STOP functions.PLoS One. 2012;7(4):e35488. doi: 10.1371/journal.pone.0035488. Epub 2012 Apr 16. PLoS One. 2012. PMID: 22523599 Free PMC article.
-
RKIP regulates bone marrow macrophage differentiation to mediate osteoclastogenesis and H-type vessel formation.Nat Commun. 2025 Aug 15;16(1):7604. doi: 10.1038/s41467-025-62972-8. Nat Commun. 2025. PMID: 40817102 Free PMC article.
-
Fixing the GAP: The role of RhoGAPs in cancer.Eur J Cell Biol. 2022 Apr;101(2):151209. doi: 10.1016/j.ejcb.2022.151209. Epub 2022 Feb 10. Eur J Cell Biol. 2022. PMID: 35180567 Free PMC article. Review.
-
FIP3-endosome-dependent formation of the secondary ingression mediates ESCRT-III recruitment during cytokinesis.Nat Cell Biol. 2012 Oct;14(10):1068-78. doi: 10.1038/ncb2577. Epub 2012 Sep 23. Nat Cell Biol. 2012. PMID: 23000966 Free PMC article.
-
Hsp70 Interacts with Mitogen-Activated Protein Kinase (MAPK)-Activated Protein Kinase 2 To Regulate p38MAPK Stability and Myoblast Differentiation during Skeletal Muscle Regeneration.Mol Cell Biol. 2018 Nov 28;38(24):e00211-18. doi: 10.1128/MCB.00211-18. Print 2018 Dec 15. Mol Cell Biol. 2018. PMID: 30275345 Free PMC article.
References
-
- Aghazadeh B., Lowry W. E., Huang X. Y., Rosen M. K. Structural basis for relief of autoinhibition of the Dbl homology domain of proto-oncogene Vav by tyrosine phosphorylation. Cell. 2000;102:625–633. - PubMed
-
- Barfod E. T., Zheng Y., Kuang W. J., Hart M. J., Evans T., Cerione R. A., Ashkenazi A. Cloning and expression of a human CDC42 GTPase-activating protein reveals a functional SH3-binding domain. J. Biol. Chem. 1993;268:26059–26062. - PubMed
-
- Barrett T., Xiao B., Dodson E. J., Dodson G., Ludbrook S. B., Nurmahomed K., Gamblin S. J., Musacchio A., Smerdon S. J., Eccleston J. F. The structure of the GTPase-activating domain from p50rhoGAP. Nature. 1997;385:458–461. - PubMed
-
- Bernards A., Settleman J. GAPs in growth factor signalling. Growth Factors. 2005;23:143–149. - PubMed
-
- Bernards A., Settleman J. GEFs in growth factor signaling. Growth Factors. 2007;25:355–361. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

