A novel model of lethal Hendra virus infection in African green monkeys and the effectiveness of ribavirin treatment
- PMID: 20660198
- PMCID: PMC2937751
- DOI: 10.1128/JVI.01163-10
A novel model of lethal Hendra virus infection in African green monkeys and the effectiveness of ribavirin treatment
Abstract
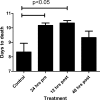
The henipaviruses, Hendra virus (HeV) and Nipah virus (NiV), are emerging zoonotic paramyxoviruses that can cause severe and often lethal neurologic and/or respiratory disease in a wide variety of mammalian hosts, including humans. There are presently no licensed vaccines or treatment options approved for human or veterinarian use. Guinea pigs, hamsters, cats, and ferrets, have been evaluated as animal models of human HeV infection, but studies in nonhuman primates (NHP) have not been reported, and the development and approval of any vaccine or antiviral for human use will likely require efficacy studies in an NHP model. Here, we examined the pathogenesis of HeV in the African green monkey (AGM) following intratracheal inoculation. Exposure of AGMs to HeV produced a uniformly lethal infection, and the observed clinical signs and pathology were highly consistent with HeV-mediated disease seen in humans. Ribavirin has been used to treat patients infected with either HeV or NiV; however, its utility in improving outcome remains, at best, uncertain. We examined the antiviral effect of ribavirin in a cohort of nine AGMs before or after exposure to HeV. Ribavirin treatment delayed disease onset by 1 to 2 days, with no significant benefit for disease progression and outcome. Together our findings introduce a new disease model of acute HeV infection suitable for testing antiviral strategies and also demonstrate that, while ribavirin may have some antiviral activity against the henipaviruses, its use as an effective standalone therapy for HeV infection is questionable.
Figures


Similar articles
-
A recombinant Hendra virus G glycoprotein subunit vaccine protects nonhuman primates against Hendra virus challenge.J Virol. 2014 May;88(9):4624-31. doi: 10.1128/JVI.00005-14. Epub 2014 Feb 12. J Virol. 2014. PMID: 24522928 Free PMC article.
-
Experimental challenge of African green monkeys with contemporary Hendra virus isolates produces divergent clinical disease.Emerg Microbes Infect. 2025 Dec;14(1):2544735. doi: 10.1080/22221751.2025.2544735. Epub 2025 Aug 22. Emerg Microbes Infect. 2025. PMID: 40768740 Free PMC article.
-
Animal challenge models of henipavirus infection and pathogenesis.Curr Top Microbiol Immunol. 2012;359:153-77. doi: 10.1007/82_2012_208. Curr Top Microbiol Immunol. 2012. PMID: 22476556 Free PMC article. Review.
-
Combined chloroquine and ribavirin treatment does not prevent death in a hamster model of Nipah and Hendra virus infection.J Gen Virol. 2010 Mar;91(Pt 3):765-72. doi: 10.1099/vir.0.017269-0. Epub 2009 Nov 4. J Gen Virol. 2010. PMID: 19889926 Free PMC article.
-
Henipavirus: a review of laboratory animal pathology.Vet Pathol. 2010 Sep;47(5):871-80. doi: 10.1177/0300985810378648. Epub 2010 Aug 3. Vet Pathol. 2010. PMID: 20682803 Review.
Cited by
-
A new model for Hendra virus encephalitis in the mouse.PLoS One. 2012;7(7):e40308. doi: 10.1371/journal.pone.0040308. Epub 2012 Jul 10. PLoS One. 2012. PMID: 22808132 Free PMC article.
-
A Survey of Henipavirus Tropism-Our Current Understanding from a Species/Organ and Cellular Level.Viruses. 2023 Oct 4;15(10):2048. doi: 10.3390/v15102048. Viruses. 2023. PMID: 37896825 Free PMC article. Review.
-
Respiratory nanoparticle-based vaccines and challenges associated with animal models and translation.J Control Release. 2015 Dec 10;219:622-631. doi: 10.1016/j.jconrel.2015.09.047. Epub 2015 Sep 26. J Control Release. 2015. PMID: 26410807 Free PMC article. Review.
-
Aggregated Hendra virus C-protein activates the NLRP3 inflammasome to induce inflammation.J Inflamm (Lond). 2023 Nov 10;20(1):38. doi: 10.1186/s12950-023-00365-8. J Inflamm (Lond). 2023. PMID: 37950278 Free PMC article.
-
Receptor-binding domains of spike proteins of emerging or re-emerging viruses as targets for development of antiviral vaccines.Emerg Microbes Infect. 2012 Aug;1(8):e13. doi: 10.1038/emi.2012.1. Epub 2012 Aug 8. Emerg Microbes Infect. 2012. PMID: 26038424 Free PMC article. Review.
References
-
- Bishop, K. A., and C. C. Broder. 2008. Hendra and Nipah: lethal zoonotic paramyxoviruses. American Society for Microbiology, Washington, DC.
-
- Bossart, K. N., Z. Zhu, D. Middleton, J. Klippel, G. Crameri, J. Bingham, J. A. McEachern, D. Green, T. J. Hancock, Y. P. Chan, A. C. Hickey, D. S. Dimitrov, L. F. Wang, and C. C. Broder. 2009. A neutralizing human monoclonal antibody protects against lethal disease in a new ferret model of acute nipah virus infection. PLoS Pathog. 5:e1000642. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

