Histone deacetylase Rpd3 regulates olfactory projection neuron dendrite targeting via the transcription factor Prospero
- PMID: 20660276
- PMCID: PMC2924735
- DOI: 10.1523/JNEUROSCI.1643-10.2010
Histone deacetylase Rpd3 regulates olfactory projection neuron dendrite targeting via the transcription factor Prospero
Abstract
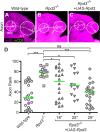
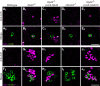
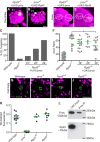
Compared to the mechanisms of axon guidance, relatively little is known about the transcriptional control of dendrite guidance. The Drosophila olfactory system with its stereotyped organization provides an excellent model to study the transcriptional control of dendrite wiring specificity. Each projection neuron (PN) targets its dendrites to a specific glomerulus in the antennal lobe and its axon stereotypically to higher brain centers. Using a forward genetic screen, we identified a mutation in Rpd3 that disrupts PN targeting specificity. Rpd3 encodes a class I histone deacetylase (HDAC) homologous to mammalian HDAC1 and HDAC2. Rpd3(-/-) PN dendrites that normally target to a dorsolateral glomerulus mistarget to medial glomeruli in the antennal lobe, and axons exhibit a severe overbranching phenotype. These phenotypes can be rescued by postmitotic expression of Rpd3 but not HDAC3, the only other class I HDAC in Drosophila. Furthermore, disruption of the atypical homeodomain transcription factor Prospero (Pros) yields similar phenotypes, which can be rescued by Pros expression in postmitotic neurons. Strikingly, overexpression of Pros can suppress Rpd3(-/-) phenotypes. Our study suggests a specific function for the general chromatin remodeling factor Rpd3 in regulating dendrite targeting in neurons, largely through the postmitotic action of the Pros transcription factor.
Figures



References
-
- Broadus J, Fuerstenberg S, Doe CQ. Staufen-dependent localization of prospero mRNA contributes to neuroblast daughter-cell fate. Nature. 1998;391:792–795. - PubMed
-
- Chihara T, Luginbuhl D, Luo L. Cytoplasmic and mitochondrial protein translation in axonal and dendritic terminal arborization. Nat Neurosci. 2007;10:828–837. - PubMed
-
- Cho Y, Griswold A, Campbell C, Min KT. Individual histone deacetylases in Drosophila modulate transcription of distinct genes. Genomics. 2005;86:606–617. - PubMed
-
- Choksi SP, Southall TD, Bossing T, Edoff K, de Wit E, Fischer BE, van Steensel B, Micklem G, Brand AH. Prospero acts as a binary switch between self-renewal and differentiation in Drosophila neural stem cells. Dev Cell. 2006;11:775–789. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
