Bacterial phenylalanine and phenylacetate catabolic pathway revealed
- PMID: 20660314
- PMCID: PMC2922514
- DOI: 10.1073/pnas.1005399107
Bacterial phenylalanine and phenylacetate catabolic pathway revealed
Abstract
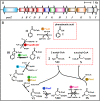
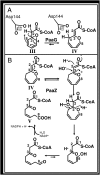
Aromatic compounds constitute the second most abundant class of organic substrates and environmental pollutants, a substantial part of which (e.g., phenylalanine or styrene) is metabolized by bacteria via phenylacetate. Surprisingly, the bacterial catabolism of phenylalanine and phenylacetate remained an unsolved problem. Although a phenylacetate metabolic gene cluster had been identified, the underlying biochemistry remained largely unknown. Here we elucidate the catabolic pathway functioning in 16% of all bacteria whose genome has been sequenced, including Escherichia coli and Pseudomonas putida. This strategy is exceptional in several aspects. Intermediates are processed as CoA thioesters, and the aromatic ring of phenylacetyl-CoA becomes activated to a ring 1,2-epoxide by a distinct multicomponent oxygenase. The reactive nonaromatic epoxide is isomerized to a seven-member O-heterocyclic enol ether, an oxepin. This isomerization is followed by hydrolytic ring cleavage and beta-oxidation steps, leading to acetyl-CoA and succinyl-CoA. This widespread paradigm differs significantly from the established chemistry of aerobic aromatic catabolism, thus widening our view of how organisms exploit such inert substrates. It provides insight into the natural remediation of man-made environmental contaminants such as styrene. Furthermore, this pathway occurs in various pathogens, where its reactive early intermediates may contribute to virulence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Fuchs G. Anaerobic metabolism of aromatic compounds. Ann N Y Acad Sci. 2008;1125:82–99. - PubMed
-
- Wang YZ, Lipscomb JD. Cloning, overexpression, and mutagenesis of the gene for homoprotocatechuate 2,3-dioxygenase from Brevibacterium fuscum. Protein Expr Purif. 1997;10:1–9. - PubMed
-
- Gibson DT, Parales RE. Aromatic hydrocarbon dioxygenases in environmental biotechnology. Curr Opin Biotechnol. 2000;11:236–243. - PubMed
-
- Boll M, Fuchs G. Benzoyl-coenzyme A reductase (dearomatizing), a key enzyme of anaerobic aromatic metabolism. ATP dependence of the reaction, purification and some properties of the enzyme from Thauera aromatica strain K172. Eur J Biochem. 1995;234:921–933. - PubMed
-
- Luengo JM, García JL, Olivera ER. The phenylacetyl-CoA catabolon: A complex catabolic unit with broad biotechnological applications. Mol Microbiol. 2001;39:1434–1442. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

