GLI2-mediated melanoma invasion and metastasis
- PMID: 20660365
- PMCID: PMC2914763
- DOI: 10.1093/jnci/djq257
GLI2-mediated melanoma invasion and metastasis
Abstract
Background: The transforming growth factor-beta (TGF-beta) pathway, which has both tumor suppressor and pro-oncogenic activities, is often constitutively active in melanoma and is a marker of poor prognosis. Recently, we identified GLI2, a mediator of the hedgehog pathway, as a transcriptional target of TGF-beta signaling.
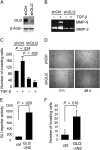
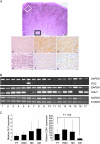
Methods: We used real-time reverse transcription-polymerase chain reaction (RT-PCR) and western blotting to determine GLI2 expression in human melanoma cell lines and subsequently classified them as GLI2high or as GLI2low according to their relative GLI2 mRNA and protein expression levels. GLI2 expression was reduced in a GLI2high cell line with lentiviral expression of short hairpin RNA targeting GLI2. We assessed the role of GLI2 in melanoma cell invasiveness in Matrigel assays. We measured secretion of matrix metalloproteinase (MMP)-2 and MMP-9 by gelatin zymography and expression of E-cadherin by western blotting and RT-PCR. The role of GLI2 in development of bone metastases was determined following intracardiac injection of melanoma cells in immunocompromised mice (n = 5-13). Human melanoma samples (n = 79) at various stages of disease progression were analyzed for GLI2 and E-cadherin expression by immunohistochemistry, in situ hybridization, or RT-PCR. All statistical tests were two-sided.
Results: Among melanoma cell lines, increased GLI2 expression was associated with loss of E-cadherin expression and with increased capacity to invade Matrigel and to form bone metastases in mice (mean osteolytic tumor area: GLI2high vs GLI2low, 2.81 vs 0.93 mm(2), difference = 1.88 mm(2), 95% confidence interval [CI] = 1.16 to 2.60, P < .001). Reduction of GLI2 expression in melanoma cells that had expressed high levels of GLI2 substantially inhibited both basal and TGF-beta-induced cell migration, invasion (mean number of Matrigel invading cells: shGLI2 vs shCtrl (control), 52.6 vs 100, difference = 47.4, 95% CI = 37.0 to 57.8, P = .024; for shGLI2 + TGF-beta vs shCtrl + TGF-beta, 31.0 vs 161.9, difference = -130.9, 95% CI = -96.2 to -165.5, P = .002), and MMP secretion in vitro and the development of experimental bone metastases in mice. Within human melanoma lesions, GLI2 expression was heterogeneous, associated with tumor regions in which E-cadherin was lost and increased in the most aggressive tumors.
Conclusion: GLI2 was directly involved in driving melanoma invasion and metastasis in this preclinical study.
Figures



Comment in
-
The ups and downs of transcription factors in melanoma.J Natl Cancer Inst. 2010 Aug 4;102(15):1103-4. doi: 10.1093/jnci/djq267. Epub 2010 Jul 21. J Natl Cancer Inst. 2010. PMID: 20660367 No abstract available.
Comment on
-
The ups and downs of transcription factors in melanoma.J Natl Cancer Inst. 2010 Aug 4;102(15):1103-4. doi: 10.1093/jnci/djq267. Epub 2010 Jul 21. J Natl Cancer Inst. 2010. PMID: 20660367 No abstract available.
References
-
- Houghton AN, Polsky D. Focus on melanoma. Cancer Cell. 2002;2(4):275–278. - PubMed
-
- Bastian BC. Molecular genetics of melanocytic neoplasia: practical applications for diagnosis. Pathology. 2004;36(5):458–461. - PubMed
-
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353(20):2135–2147. - PubMed
-
- Crowson AN, Magro C, Miller A, Mihm MC., Jr. The molecular basis of melanomagenesis and the metastatic phenotype. Semin Oncol. 2007;34(6):476–490. - PubMed
-
- Smalley KS. A pivotal role for ERK in the oncogenic behaviour of malignant melanoma? Int J Cancer. 2003;104(5):527–532. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

