Tumor pH and its measurement
- PMID: 20660380
- PMCID: PMC4351768
- DOI: 10.2967/jnumed.109.068981
Tumor pH and its measurement
Abstract
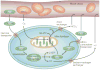
Studies over the last few decades have demonstrated that the intracellular pH of solid tumors is maintained within a range of 7.0-7.2, whereas the extracellular pH is acidic. A low extracellular pH may be an important factor inducing more aggressive cancer phenotypes. Research into the causes and consequences of this acidic pH of tumors is highly dependent on accurate, precise, and reproducible measurements, and these have undergone great changes in the last decade. This review focuses on the most recent advances in the in vivo measurement of tumor pH by pH-sensitive PET radiotracers, MR spectroscopy, MRI, and optical imaging.
Figures

References
-
- Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nature Reviews Cancer. 2004 Nov;4(11):891–899. - PubMed
-
- Gillies RJ, Morse DL. In vivo magnetic resonance spectroscopy in cancer. Annual review of biomedical engineering. 2005;7:287–326. - PubMed
-
- Gallagher FA, Kettunen MI, Day SE, et al. Magnetic resonance imaging of pH in vivo using hyperpolarized 13C-labelled bicarbonate. Nature. 2008 Jun 12;453(7197):940–943. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
