Impaired binding of the age-related macular degeneration-associated complement factor H 402H allotype to Bruch's membrane in human retina
- PMID: 20660596
- PMCID: PMC2943316
- DOI: 10.1074/jbc.M110.103986
Impaired binding of the age-related macular degeneration-associated complement factor H 402H allotype to Bruch's membrane in human retina
Abstract
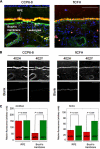
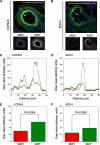
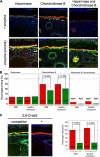
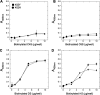
Age-related macular degeneration (AMD) is the predominant cause of blindness in the industrialized world where destruction of the macula, i.e. the central region of the retina, results in loss of vision. AMD is preceded by the formation of deposits in the macula, which accumulate between the Bruch's membrane and the retinal pigment epithelium (RPE). These deposits are associated with complement-mediated inflammation and perturb retinal function. Recent genetic association studies have demonstrated that a common allele (402H) of the complement factor H (CFH) gene is a major risk factor for the development of AMD; CFH suppresses complement activation on host tissues where it is believed to bind via its interaction with polyanionic structures. We have shown previously that this coding change (Y402H; from a tyrosine to histidine residue) alters the binding of the CFH protein to sulfated polysaccharides. Here we demonstrate that the AMD-associated polymorphism profoundly affects CFH binding to sites within human macula. Notably, the AMD-associated 402H variant binds less well to heparan sulfate and dermatan sulfate glycosaminoglycans within Bruch's membrane when compared with the 402Y form; both allotypes exhibit a similar level of binding to the RPE. We propose that the impaired binding of the 402H variant to Bruch's membrane results in an overactivation of the complement pathway leading to local chronic inflammation and thus contributes directly to the development and/or progression of AMD. These studies therefore provide a putative disease mechanism and add weight to the genetic association studies that implicate the 402H allele as an important risk factor in AMD.
Figures

References
-
- Hageman G. S., Luthert P. J., Victor Chong N. H., Johnson L. V., Anderson D. H., Mullins R. F. (2001) Prog. Retin. Eye Res. 20, 705–732 - PubMed
-
- Day A. J., Willis A. C., Ripoche J., Sim R. B. (1988) Immunogenetics 27, 211–214 - PubMed
-
- Edwards A. O., Ritter R., 3rd, Abel K. J., Manning A., Panhuysen C., Farrer L. A. (2005) Science 308, 421–424 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

